Alert-v5.0(2024EN)
General information
Name: nl.zorg.Alert ![]()
Version: 5.0
HCIM Status:Final
Release: 2024
Release status: Published
Release date: 24-04-2024
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 15-12-2014 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.8.3 |
| DCM::KeywordList | alerts, alert, waarschuwing |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.Alert |
| DCM::PublicationDate | 24-04-2024 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 07-04-2025 |
| DCM::Supersedes | nl.zorg.Alert-v4.2 |
| DCM::Version | 5.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (01-04-2015)
| ZIB-109 | Onduidelijk wat wel/niet onderdeel uitmaakt van bouwsteen OverdrachtAlert; definities aanpassen |
| ZIB-132 | Aanpassingen in SNOMED CT codes en omschrijvingen voor codelijst AlertTypeCodelijst n.a.v. review terminologie expert. |
| ZIB-203 | In de klinische bouwsteen OverdrachtAlert DCM::ValueSet SNOMED CT aangepast van concept AlertOmschrijving naar tagged value DCM::ContentExpression. |
| ZIB-204 | In de klinische bouwsteen OverdrachtAlert DCM::ValueSet G-Standaard aangepast van concept AlertOmschrijving. |
| ZIB-306 | Aanpassen modellering keuzebox, boundary en cardinaliteit |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
| ZIB-352 | Opsplitsen bouwsteen OverdrachtAlert |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 3.0 (01-05-2016)
| ZIB-438 | Geen informatie |
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
| ZIB-574 | Alleen verwijzen naar het rootconcept van de ZIB. |
Publicatieversie 3.1 (04-09-2017)
| ZIB-546 | Toevoegen ' Melding kindermishandeling' aan het waardenlijst AlertnaamCodelijst |
Publicatieversie 3.2 (31-12-2017)
| ZIB-593 | Herzie Alert codelijsten |
Publicatieversie 3.3 (26-02-2019)
| ZIB-682 | toelichting toevoegen aan alert |
Publicatieversie 3.4 (06-07-2019)
| ZIB-813 | AlertNaamCodelijst uitbreiden met dragerschap van multiresistente bacteriën |
Publicatieversie 4.0 (31-01-2020)
| ZIB-905 | Typo: vasrgelegd bij Alert |
| ZIB-526 | Toevoegen EindDatum bij zibs die ook BeginDatum kennen |
Publicatieversie 4.1 (01-09-2020)
| ZIB-1160 | Typo in wiki zib Alert 4.0 |
| ZIB-1209 | Tekstueel en in voorbeelden aanpassen zib Alert vanwege de nieuwe zib CI |
Publicatieversie 4.2 (15-04-2024)
| ZIB-1440 | Omschrijving BeginDatum verbeteren, +bijkomende verbeteringen elementen |
| ZIB-1769 | Aanpassing zib MedicatieContraIndicatie/Alert |
Publicatieversie 5.0 (24-04-2024)
| ZIB-2632 | Alert - Vervanging van de verwijzing naar Probleem |
| ZIB-2679 | Alert - Evidence base: Functionaliteit (informatief) verwijderen |
| ZIB-2735 | Pre-publicatie check op deprecated codes |
Concept
An alert describes a clinical or administrative fact brought to the attention of the users of the clinical systems to be taken into account when shaping diagnostic and therapeutic policy or in dealing with the patient, usually because of a safety risk.
This zib is not intended to specify hypersensitivities or intolerances for a specific substance or group of substances. Monitoring for this can be represented on the basis of the zib SurveillanceDecision.
Examples of warnings:
- A disorder, condition or diagnosis which can be considered as a contraindication for undergoing a certain type of therapy, such as pregnancy or long QT syndrome;
- Impaired functioning of an organ system (heart failure, impaired liver or kidney function, weakened immune system);
- Risk of spreading certain microorganisms (multi-resistant bacteria, tubercle bacilli, HIV, HBV, Ebola virus);
- Other risks.
Purpose
Documenting and entering disorders or conditions that require attention is an important part of medical registration. It concerns the core of patient safety. In the execution of research and treatment, these patient characteristics - which are marked as a warning - constantly have to be taken into account. They provide information that is important for the patient’s condition and the options a healthcare provider has for therapy. Patient characteristics that are registered or transferred as an Alert can also be described as a Diagnosis or HypersensitivityIntolerance. The difference is in the fact that the healthcare provider considers the diagnoses, hypersensitivity or intolerance as an Alert = warning. In many cases, transfer will be subject to strict privacy rules, as the warning will not always elicit an adequate reaction in the informed environment.
Medication monitoring based on potential medication contraindications is based on non-patient-specific pharmacological characteristics of medicines. The zib Alert is not intended for medication monitoring based on specific substances to which one patient may react adversely while another does not. For this form of medication monitoring, the zib SurveillanceDecision is intended.
Evidence Base
The zib Alert covers a wide range of patient characteristics based on which the health professional wants to receive unconditional or conditional warnings. This concerns warnings regarding certain infections that require specific (isolation) measures, situations that may be a contraindication for certain treatments or examinations (such as a pacemaker for MRI examinations) or characteristics that may be a contraindication for certain medicines. This latter category 'possible contraindication for medicine' can concern a disorder or condition, but also behavior (such as being a top athlete) or desire to have children.
The MedicationContraindicationNameCodelist contains values from the G-standard Contraindications (Thesaurus 40), for medication surveillance.
Information Model
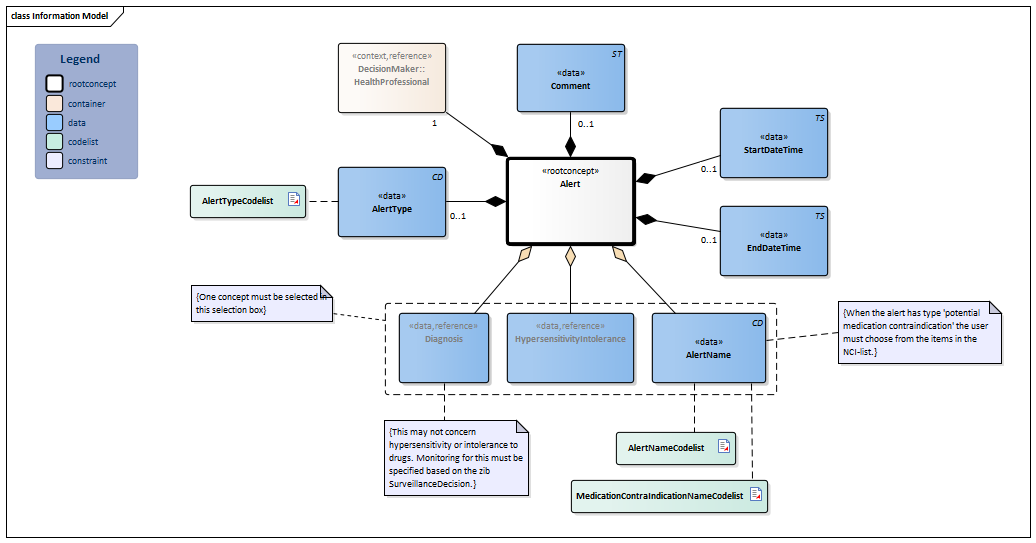
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||
| NL-CM:8.3.1 | Root concept of the Alert information model. This root concept contains all data elements of the Alert information model. |
|
|||||||||||||
| NL-CM:8.3.5 | 0..1 | The date and time at which the described condition was entered as a warning.
This can be an exact date and time, or a rough indication of the date (such as only the year, or the month and the year). |
|||||||||||||
| NL-CM:8.3.8 | 0..1 | The date and time at which the described condition was retracted as a warning.
This can be an exact date and time, or a rough indication of the date (such as only the year, or the month and the year). |
|||||||||||||
| NL-CM:8.3.10 | (0..1) | A warning about a particular diagnosis, because it may pose a risk to the patient with certain treatments. For example, 'Pacemaker' can be included as an alert. |
| ||||||||||||
| NL-CM:8.3.11 | (0..1) | A warning for a specific hypersensitivity or intolerance, because this can pose a risk to the patient with certain treatments. For example, an alert can be given such as 'Hypersensitivity to UV light'. |
| ||||||||||||
| NL-CM:8.3.4 | (0..1) | A warning, other than a condition or problem. For example, a patient can be given an ‘Aggressive patient' alert.
The warning can be entered in code (there are codes for frequently used alerts), but seeing the dynamic nature of the warnings cf. SARS and Ebola, these alerts will often be entered as free text. |
| ||||||||||||
| NL-CM:8.3.6 | 0..1 | Indicates the type of alert, meaning a rough description of the cause or origin of the warning. |
| ||||||||||||
| NL-CM:8.3.9 | 1 | The health professional who is responsible for setting the alert. |
|
| |||||||||||
| NL-CM:8.3.7 | 0..1 | Explanatory comments to the alert that can not be expressed in any of the other elements. |
|
||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| Alert | |
| AlertNaam | Zwangerschap |
| AlertType | Conditie |
| BeginDatumTijd | 15-11-2022 |
| EindDatumTijd | 10-08-2023 |
| RedenBeëindigingAlert | Einde zwangerschap |
| Vaststeller::Zorgverlener | |
| Naam | R. van der Laan - Bakker |
| Specialisme | Verloskundige |
| Alert | |
| AlertNaam | Drager MRSA |
| AlertType | Waarschuwing |
| BeginDatumTijd | 12-01-2024 |
| Vaststeller::Zorgverlener | |
| Naam | G. de Zeeuw |
| Specialisme | Microbioloog |
| Alert | |
| AlertNaam | Topsportbeoefening |
| AlertType | Mogelijke medicatie contra-indicatie |
| BeginDatumTijd | 18-10-2020 |
| Vaststeller::Zorgverlener | |
| Naam | L. Peeters |
| Specialisme | Sportgeneeskunde |
Instructions
The Alerts of the type “possible contraindication for medication” are intended to be used (alongside SurveillanceDecision), to determine whether a warning is necessary.
Valuesets
AlertNameCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.3.2 | Binding: Extensible | Status: Active |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Carrier of infectious organism | 66598005 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager van besmettelijke ziekte |
| Carrier of extended spectrum beta-lactamase producing bacteria | 762988003 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager van ESBL-producerende bacterie |
| Carbapenemase producing Enterobacteriaceae carrier | 715881003 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Enterobacteriaceae – CPE |
| Multidrug-resistant bacteria carrier | 430381000146105 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager van BRMO – Algemeen |
| Carrier of carbapenem susceptible multidrug resistant Enterobacteriaceae | 97961000146102 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Enterobacteriaceae – BRMO excl. CPE |
| Carrier of multiple drug resistant Stenotrophomonas maltophilia | 97981000146105 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Stenotrophomonas maltophilia – BRMO |
| Carrier of multiple drug resistant Acinetobacter | 97971000146108 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Acinetobacter spp – BRMO |
| Carrier of multiple drug resistant Pseudomonas aeruginosa | 98001000146104 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Pseudomonas aeruginosa – BRMO |
| Carrier of vancomycin resistant enterococcus | 431109006 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Enterococcus faecium – VRE |
| Carrier of multiple drug resistant Streptococcus pneumoniae | 97991000146107 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager Streptococcus pneumoniae – PRP |
| Carrier of methicillin resistant Staphylococcus aureus | 432415000 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager MRSA |
| Human immunodeficiency virus carrier | 699433000 | SNOMED CT | 2.16.840.1.113883.6.96 | Drager HIV |
| Victim of elder abuse | 706872008 | SNOMED CT | 2.16.840.1.113883.6.96 | Slachtoffer van ouderenmishandeling |
| Victim of child abuse | 397940009 | SNOMED CT | 2.16.840.1.113883.6.96 | Kindermishandeling |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||||
AlertTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.3.1 | Binding: Required | Status: Active |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Condition | 75323-6 | LOINC | 2.16.840.1.113883.6.1 | conditie |
| Potential contraindication for medication | 350241000146102 | SNOMED CT | 2.16.840.1.113883.6.96 | mogelijke contra-indicatie voor geneesmiddel |
| Alert | 74018-3 | LOINC | 2.16.840.1.113883.6.1 | waarschuwing |
| Other values are not allowed | ||||
MedicationContraIndicationNameCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.3.3 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Contra Indicaties (Thesaurus 40) | 2.16.840.1.113883.2.4.4.1.902.40 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
This information model in other releases
- Release 2015, (Version 1.0)
- Release 2016, (Version 3.0)
- Release 2017, (Version 3.2)
- Prerelease 2018-2, (Version 3.3)
- Prerelease 2019-2, (Version 4.0)
- Release 2020, (Version 4.1)
- Prerelease 2021-2, (Version 4.1)
- Prerelease 2022-1, (Version 4.1)
- Prerelease 2023-1, (Version 4.1)
- Prerelease 2024-1, (Versie 4.2)
Information model references
This information model refers to
This information model is used in
- --
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release 2024
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms versie: 20250430 [R] (april 2025-editie)
- LOINC version 2.77
Conditions for use are located on the mainpage ![]()
This page is generated on 27/05/2025 13:57:46 with ZibExtraction v. 9.5.9242.40707