SurveillanceDecision-v1.1(2024EN): verschil tussen versies
Nieuwe pagina aangemaakt met '<!-- Hieronder wordt een transclude page aangeroepen --> {{Versions-2.16.840.1.113883.2.4.3.11.60.40.3.8.5(EN)|1|SurveillanceDecision-v1.1(2024EN)}} <!-- Tot hier de transclude page --> ==General information<!--hdGeneralInformation-->== Name<!--hdName-->: '''nl.zorg.SurveillanceDecision''' link=BewakingBesluit-v1.1(2024NL)<BR> Version<!--hdVersion-->: '''1.1''' <br> HCIM Status<!--hdStatus-->:Final<br> Release<!--hdPublication-->: '''…' |
Geen bewerkingssamenvatting |
||
| Regel 8: | Regel 8: | ||
Release<!--hdPublication-->: '''2024''' <br> | Release<!--hdPublication-->: '''2024''' <br> | ||
Release status<!--hdPublicationStatus-->: Published<br> | Release status<!--hdPublicationStatus-->: Published<br> | ||
Release date<!--hdPublicationDate-->: | Release date<!--hdPublicationDate-->: 24-04-2024 | ||
<!-- Aanroep Errata transclude page --> | <!-- Aanroep Errata transclude page --> | ||
{{ErrataEN<!--hdErrata-->|2024|{{PAGENAME}}}} | {{ErrataEN<!--hdErrata-->|2024|{{PAGENAME}}}} | ||
| Regel 50: | Regel 50: | ||
|style="width:250px; "|DCM::Name||nl.zorg.BewakingBesluit | |style="width:250px; "|DCM::Name||nl.zorg.BewakingBesluit | ||
|- | |- | ||
|style="width:250px; "|DCM::PublicationDate|| | |style="width:250px; "|DCM::PublicationDate||24-04-2024 | ||
|- | |- | ||
|style="width:250px; "|DCM::PublicationStatus||Published | |style="width:250px; "|DCM::PublicationStatus||Published | ||
| Regel 73: | Regel 73: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_2#ZIB-1340 | ZIB-1340 ]] | |style="width:75px; "|[[ZIBIssues500_2#ZIB-1340 | ZIB-1340 ]] | ||
| | |Ontkenning van een overgevoeligheid In AllergieIntolerantie | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_2#ZIB-1440 | ZIB-1440 ]] | |style="width:75px; "|[[ZIBIssues500_2#ZIB-1440 | ZIB-1440 ]] | ||
| | |Omschrijving BeginDatum verbeteren, +bijkomende verbeteringen elementen | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_3#ZIB-1986 | ZIB-1986 ]] | |style="width:75px; "|[[ZIBIssues500_3#ZIB-1986 | ZIB-1986 ]] | ||
| | |Zib Overgevoeligheid- naam Stof wijzigen in TeBewakenStof | ||
|} | |} | ||
Publicatieversie <u>1.1</u> ( | Publicatieversie <u>1.1</u> (24-04-2024) | ||
{| | {| | ||
|- | |- | ||
| Regel 749: | Regel 749: | ||
SNOMED CT and LOINC codes are based on: | SNOMED CT and LOINC codes are based on: | ||
<ul> | <ul> | ||
<li>SNOMED Clinical Terms versie: | <li>SNOMED Clinical Terms versie: 20250430 [R] (april 2025-editie)</li> | ||
<li>LOINC version 2.77</li> | <li>LOINC version 2.77</li> | ||
</ul> | </ul> | ||
Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | ||
This page is generated on | This page is generated on 27/05/2025 13:58:03 with ZibExtraction v. 9.5.9242.40707<!--ftDate--> <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2024(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2024(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2024(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2024(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | ||
Huidige versie van 27 mei 2025 om 16:55
General information
Name: nl.zorg.SurveillanceDecision ![]()
Version: 1.1
HCIM Status:Final
Release: 2024
Release status: Published
Release date: 24-04-2024
Metadata
| DCM::CoderList | Zib-centrum |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Zib-centrum |
| DCM::CreationDate | 07-06-2023 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | * |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.8.5 |
| DCM::KeywordList | |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | * |
| DCM::Name | nl.zorg.BewakingBesluit |
| DCM::PublicationDate | 24-04-2024 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Zib-centrum |
| DCM::RevisionDate | 02-04-2025 |
| DCM::Supersedes | nl.zorg.BewakingBesluit-v1.0 |
| DCM::Version | 1.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-04-2024)
| ZIB-1340 | Ontkenning van een overgevoeligheid In AllergieIntolerantie |
| ZIB-1440 | Omschrijving BeginDatum verbeteren, +bijkomende verbeteringen elementen |
| ZIB-1986 | Zib Overgevoeligheid- naam Stof wijzigen in TeBewakenStof |
Publicatieversie 1.1 (24-04-2024)
| ZIB-2454 | Bewakingsbesluit - Engelse benaming voor concept BesluitReden veranderen naar DecisionReason en codelijsten hernoemen conform naamconventie. |
| ZIB-2634 | BewakingBesluit - BesluitReden wordt vrije tekst |
| ZIB-2642 | BewakingBesluit - Voorbeeld maken van Veilig binnen Onveilige groep |
| ZIB-2680 | BewakingBesluit - Evidence base: Functionaliteit (informatief) verwijderen |
Concept
The decision to initiate or terminate surveillance for a substance or group of substances that may cause an adverse reaction in the patient.
Purpose
Specifying a surveillance decision with an effective date makes it clear on which substance or group of substances surveillance has started or stopped. An overview of surveillance decisions provides insight into which substances are subject to surveillance and options for managing surveillance decisions.
Evidence Base
The zib SurveillanceDecision represents the health professional’s decision to start or end surveillance of a substance or group of substances. Initiating surveillance means that the health professional wants to receive a warning if an unsafe substance is prescribed. The DecisionEffectiveDateTime represents the moment at which surveillance should start or end, depending on the DecisionType (started or discontinued).
The user can indicate the reason for starting a surveillance decision with one of the three following options:
- A hypersensitivity or intolerance: to be indicated via a reference to HypersensitivityIntolerance
- A reaction: to be indicated via a reference to Reaction
- Specify a decision reason in free text.
The SafeWithinUnsafeGroup element makes it possible to make exceptions within a (large) group of unsafe substances. Suppose that 78 of a group of substances are unsafe and 2 are safe, then one does not need to record a surveillance decision for each of the 78 unsafe substances. Instead, it will suffice to record one surveillance decision in which the entire group of 80 substances is declared as unsafe and 2 substances that are safe within that group.
Where a reaction is related to a (component of) a substance actually administered, the health professional may decide to start surveillance for a broader collection of substances. The substance(s) indicated in a surveillance decision may therefore differ from the substance to which the patient has actually developed a reaction.
A hypersensitivity or intolerance involves a diagnosis in which it has been demonstrated or assumed that the patient has a tendency to develop an adverse reaction when exposed to a certain substance or group of substances. In most cases, a surveillance decision related to this hypersensitivity or intolerance will concern the same substances, but not necessarily. Additional diagnostiscs may reveal an allergy based on a limited number of actually tested substances, and it may then be decided to start surveillance for the entire group to which those substances belong.
Even if no additional diagnostics are possible, but an intolerance is suspected, the specification of the substance(s) for which the health professional decides to start surveillance may deviate from the substance(s) that are recorded for the hypersensitivity or intolerance.
Information Model
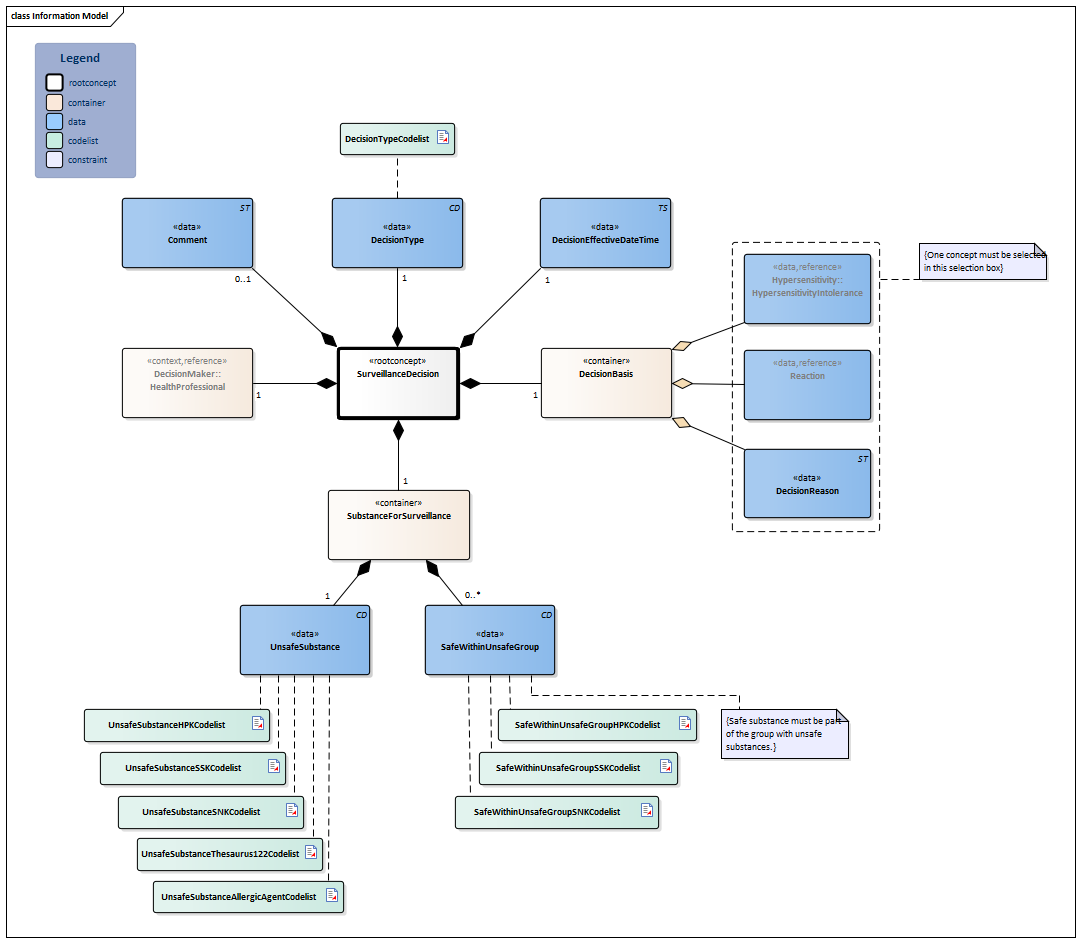
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | ||||||||||||||||
| NL-CM:8.5.1 | This is a reference to the root concept of information model SurveillanceDecision. |
|
||||||||||||||||||||
| NL-CM:8.5.2 | 1 | The kind of decision: to start or stop the surveillance. |
|
| ||||||||||||||||||
| NL-CM:8.5.3 | 1 | Moment (date and time) when the decision should take effect. |
|
|||||||||||||||||||
| NL-CM:8.5.4 | 1 | Container of the DecisionBasis concept.This container contains all data elements of the DecisionBasis concept.
This concerns the basis for the surveillance decision to start or end the surveillance. |
||||||||||||||||||||
| NL-CM:8.5.5 | (0..1) | Hypersensitivity in the patient as reason for the surveillance decision. |
|
| ||||||||||||||||||
| NL-CM:8.5.6 | (0..1) | Reaction in the patient as reason for the surveillance decision. |
|
| ||||||||||||||||||
| NL-CM:8.5.7 | (0..1) | Reason for the surveillance decision: to start or end the surveillance. |
|
|||||||||||||||||||
| NL-CM:8.5.8 | 1 | Container of the SubstanceForSurveillance concept. This container contains all data elements of the SubstanceForSurveillance concept.
Substance or group of substances that must be monitored in the sense that prescription will trigger a message. |
|
|||||||||||||||||||
| NL-CM:8.5.9 | 1 | The substance or group of substances that must be monitored completely or with exceptions. |
|
| ||||||||||||||||||
| NL-CM:8.5.10 | 0..* | Exception within the group of substances to be monitored that do not require monitoring. |
|
| ||||||||||||||||||
| NL-CM:8.5.12 | 1 | The health professional who made the surveillance decision. |
|
| ||||||||||||||||||
| NL-CM:8.5.13 | 0..1 | Textual explanation of the surveillance decision which cannot be expressed in any of the other fields. |
|
|||||||||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| BewakingBesluit | |
| BesluitType | Bewaking gestart |
| BesluitIngangsDatumTijd | 02-07-2023 |
| TeBewakenStof | |
| OnveiligeStof | Valproïnezuur |
| VeiligBinnenOnveiligeGroep | |
| Toelichting | Gegevens in verwijsbrief van huisarts. |
| Besluitgrond | |
| BesluitReden | Stof heeft (mogelijk) een nadelige reactie veroorzaakt. |
| Beslisser::Zorgverlener | |
| Naam | R. Verhagen-De Leeuw |
| Specialisme | Huisartsgeneeskunde |
| BewakingBesluit | |
| BesluitType | Bewaking gestart |
| BesluitIngangsDatumTijd | 05-04-2024 |
| TeBewakenStof | |
| OnveiligeStof | Cefalosporines |
| VeiligBinnenOnveiligeGroep 1 | Cefalozine |
| VeiligBinnenOnveiligeGroep 2 | Ceftriaxon |
| VeiligBinnenOnveiligeGroep 3 | Ceftazidim |
| Toelichting | |
| Besluitgrond | |
| BesluitReden | Gedeeltelijke kruisovergevoeligheid met Cefalosporines. |
| Beslisser::Zorgverlener | |
| Naam | G.L. Den Hartog |
| Specialisme | Inwendige geneeskunde |
| BewakingBesluit | |
| BesluitType | Bewaking gestart |
| BesluitIngangsDatumTijd | 12-09-2023 14:30 |
| TeBewakenStof | |
| OnveiligeStof | Heparine |
| VeiligBinnenOnveiligeGroep | |
| Toelichting | Antistolling na CABG |
| Reactie | |
| ReactieNaam | Heparine-geïnduceerde trombocytopenie |
| Beslisser::Zorgverlener | |
| Naam | J. Gielissen |
| Specialisme | Hematologie |
| BewakingBesluit | |||
| BesluitType | Bewaking gestart | Bewaking gestopt | Bewaking gestart |
| BesluitIngangsDatumTijd | 17-03-2018 | 02-01-2024 | 02-01-2024 |
| TeBewakenStof | |||
| OnveiligeStof | Penicillines | Penicillines | Amoxicilline/clavulaanzuur |
| VeiligBinnenOnveiligeGroep | |||
| Besluitgrond | |||
| BesluitReden | Stof heeft (mogelijk) een nadelige reactie veroorzaakt. | De groep waarop wordt bewaakt, is groter of kleiner dan nodig. | Stof heeft (mogelijk) een nadelige reactie veroorzaakt. |
| Toelichting | Patiënte werd erg misselijk en had extreme diarree. Wil het middel nooit meer gebruiken. | Ceftriaxon werd goed verdragen, waarschijnlijk reactie gehad op amoxiclav. | |
| Beslisser::Zorgverlener | |||
| Naam | F. Zegers | G.J. Zaal | G.J. Zaal |
| Specialisme | Huisartsgeneeskunde | SEH-arts | SEH-arts |
Instructions
Medication surveillance with regard to medication contraindication is based on the Zib Alert with AlertType 'Possible medication contraindication'
Valuesets
DecisionTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.1 | Binding: Required | Status: Active |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Started | 385652002 | SNOMED CT | 2.16.840.1.113883.6.96 | Bewaking gestart |
| Discontinued | 410546004 | SNOMED CT | 2.16.840.1.113883.6.96 | Bewaking gestopt |
| Other values are not allowed | ||||
SafeWithinUnsafeGroupHPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.5 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
SafeWithinUnsafeGroupSNKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.7 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Stofnaamcode (SNK) | 2.16.840.1.113883.2.4.4.1.750 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
SafeWithinUnsafeGroupSSKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.10 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Stofnaamcode i.c.m. toedieningsweg (SSK) | 2.16.840.1.113883.2.4.4.1.725 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
UnsafeSubstanceAllergicAgentCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.4 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: ^98061000146100|Dutch total non-drug allergen simple reference set| | SNOMED CT | 2.16.840.1.113883.6.96 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
UnsafeSubstanceHPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.6 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
UnsafeSubstanceSNKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.8 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Stofnaamcode (SNK) | 2.16.840.1.113883.2.4.4.1.750 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
UnsafeSubstanceSSKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.9 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Stofnaamcode i.c.m. toedieningsweg (SSK) | 2.16.840.1.113883.2.4.4.1.725 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
UnsafeSubstanceThesaurus122Codelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.8.5.11 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Ongewenste medicatiegroepen | 2.16.840.1.113883.2.4.4.1.902.122 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
This information model in other releases
Information model references
This information model refers to
This information model is used in
- --
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release 2024
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms versie: 20250430 [R] (april 2025-editie)
- LOINC version 2.77
Conditions for use are located on the mainpage ![]()
This page is generated on 27/05/2025 13:58:03 with ZibExtraction v. 9.5.9242.40707