PharmaceuticalProduct-v2.2.1(2024EN): verschil tussen versies
Nieuwe pagina aangemaakt met '<!-- Hieronder wordt een transclude page aangeroepen --> {{Versions-2.16.840.1.113883.2.4.3.11.60.40.3.9.7(EN)|1|PharmaceuticalProduct-v2.2.1(2024EN)}} <!-- Tot hier de transclude page --> ==General information<!--hdGeneralInformation-->== Name<!--hdName-->: '''nl.zorg.part.PharmaceuticalProduct''' link=FarmaceutischProduct-v2.2.1(2024NL)<BR> Version<!--hdVersion-->: '''2.2.1''' <br> HCIM Status<!--hdStatus-->:Final<br> Release<!--hdP...' |
Geen bewerkingssamenvatting |
||
| Regel 7: | Regel 7: | ||
HCIM Status<!--hdStatus-->:Final<br> | HCIM Status<!--hdStatus-->:Final<br> | ||
Release<!--hdPublication-->: '''2024''' <br> | Release<!--hdPublication-->: '''2024''' <br> | ||
Release status<!--hdPublicationStatus-->: | Release status<!--hdPublicationStatus-->: Published<br> | ||
Release date<!--hdPublicationDate-->: | Release date<!--hdPublicationDate-->: 23-04-2025 | ||
<!-- Aanroep Errata transclude page --> | <!-- Aanroep Errata transclude page --> | ||
{{ErrataEN<!--hdErrata-->|2024|{{PAGENAME}}}} | {{ErrataEN<!--hdErrata-->|2024|{{PAGENAME}}}} | ||
| Regel 50: | Regel 50: | ||
|style="width:250px; "|DCM::Name||nl.zorg.part.FarmaceutischProduct | |style="width:250px; "|DCM::Name||nl.zorg.part.FarmaceutischProduct | ||
|- | |- | ||
|style="width:250px; "|DCM::PublicationDate|| | |style="width:250px; "|DCM::PublicationDate||23-04-2025 | ||
|- | |- | ||
|style="width:250px; "|DCM::PublicationStatus|| | |style="width:250px; "|DCM::PublicationStatus||Published | ||
|- | |- | ||
|style="width:250px; "|DCM::ReviewerList||Projectgroep Medicatieproces & | |style="width:250px; "|DCM::ReviewerList||Projectgroep Medicatieproces & Architectuurgroep Registratie aan de Bron | ||
|- | |- | ||
|style="width:250px; "|DCM::RevisionDate||13-09-2023 | |style="width:250px; "|DCM::RevisionDate||13-09-2023 | ||
| Regel 85: | Regel 85: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-781 | ZIB-781 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-781 | ZIB-781 ]] | ||
|aanpassing omschrijving | |aanpassing omschrijving 'Farmaceutische vorm' | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-788 | ZIB-788 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-788 | ZIB-788 ]] | ||
| Regel 91: | Regel 91: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-795 | ZIB-795 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-795 | ZIB-795 ]] | ||
|aanpassing voorbeeld | |aanpassing voorbeeld 'Product (geneesmiddel)' | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-796 | ZIB-796 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-796 | ZIB-796 ]] | ||
|aanpassing definitie | |aanpassing definitie 'ProductCode (geneesmiddelcode)' | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-797 | ZIB-797 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-797 | ZIB-797 ]] | ||
|aanpassing definitie | |aanpassing definitie 'Product (geneesmiddel)' | ||
|} | |} | ||
| Regel 104: | Regel 104: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-789 | ZIB-789 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-789 | ZIB-789 ]] | ||
|aanpassing voorbeeld en omschrijving | |aanpassing voorbeeld en omschrijving 'Ingredientcode' (in zib FarmaceutischProduct) | ||
|} | |} | ||
| Regel 111: | Regel 111: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_1#ZIB-911 | ZIB-911 ]] | |style="width:75px; "|[[ZIBIssues500_1#ZIB-911 | ZIB-911 ]] | ||
|Correction English | |Correction English "Farmaceutical" | ||
|} | |} | ||
| Regel 118: | Regel 118: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_2#ZIB-1452 | ZIB-1452 ]] | |style="width:75px; "|[[ZIBIssues500_2#ZIB-1452 | ZIB-1452 ]] | ||
| | |Geen informatie | ||
|} | |} | ||
| Regel 125: | Regel 125: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_2#ZIB-1453 | ZIB-1453 ]] | |style="width:75px; "|[[ZIBIssues500_2#ZIB-1453 | ZIB-1453 ]] | ||
| | |Geen informatie | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_3#ZIB-1651 | ZIB-1651 ]] | |style="width:75px; "|[[ZIBIssues500_3#ZIB-1651 | ZIB-1651 ]] | ||
| | |Geen informatie | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_3#ZIB-1726 | ZIB-1726 ]] | |style="width:75px; "|[[ZIBIssues500_3#ZIB-1726 | ZIB-1726 ]] | ||
| | |Geen informatie | ||
|} | |} | ||
| Regel 138: | Regel 138: | ||
|- | |- | ||
|style="width:75px; "|[[ZIBIssues500_3#ZIB-1743 | ZIB-1743 ]] | |style="width:75px; "|[[ZIBIssues500_3#ZIB-1743 | ZIB-1743 ]] | ||
| | |Geen informatie | ||
|} | |} | ||
</div> | </div> | ||
| Regel 212: | Regel 212: | ||
|Coding medication in the Netherlands is done on the basis of the G standard (issued by Z index), which is filled under the direction of KNMP. | |Coding medication in the Netherlands is done on the basis of the G standard (issued by Z index), which is filled under the direction of KNMP. | ||
The coded medication can be expressed as: | The coded medication can be expressed as: | ||
<ul> | |||
<li>Global Trade Item Number (GTIN)</li> | |||
<li>Z index number (ZI number)</li> | |||
<li>Trade product code (HPK)</li> | |||
<li>Prescription code (PRK)</li> | |||
<li>Generic product code (GPK)</li> | |||
<li>Anatomic Therapeutic Classification code (ATC)</li> | |||
</ul> | |||
The GTIN enables identification of the product including its origin with a barcode. | The GTIN enables identification of the product including its origin with a barcode. | ||
The ZI number represents the trade product and the package size. | The ZI number represents the trade product and the package size. | ||
| Regel 272: | Regel 272: | ||
|colspan ="4" style ="padding-left: 0px"|<span Id=2771 Title="NL: SerieNummer">[[Bestand: arrowright.png | 10px | link=]]SerieNumber</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=2771 Title="NL: SerieNummer">[[Bestand: arrowright.png | 10px | link=]]SerieNumber</span> | ||
|0..1 | |0..1 | ||
|The serial number uniquely identifies the source of information of the dose itself. The number is constructed according to the GS1 standard AI(21). ( | |The serial number uniquely identifies the source of information of the dose itself. The number is constructed according to the GS1 standard AI(21). (<A title="Volg link" href="https://www.gs1.nl/sectorafspraken-over-standaarden/unieke-identificatie-en-productdata-gezondheidszorg/gs1-barcodes-2#application-identifiers-ais">https://www.gs1.nl/sectorafspraken-over-standaarden/unieke-identificatie-en-productdata-gezondheidszorg/gs1-barcodes-2#application-identifiers-ais</A>) | ||
| | | | ||
| | | | ||
| Regel 335: | Regel 335: | ||
|Active substance or excipient. | |Active substance or excipient. | ||
Here, the same codes can be used as for the ProductCode (for dilutions and compounds in particular), but now, the SSK and SNK codes can also be used to indicate a substance (to list ingredients of local products prepared by the pharmacy). | Here, the same codes can be used as for the ProductCode (for dilutions and compounds in particular), but now, the SSK and SNK codes can also be used to indicate a substance (to list ingredients of local products prepared by the pharmacy). | ||
<ul> | |||
<li>GTIN International Article Number </li> | |||
<li>Z-index number (ZI-number) </li> | |||
<li>Trade product code (HPK) </li> | |||
<li>Prescription code (PRK) </li> | |||
<li>Generic product code (GPK) </li> | |||
<li>Anatomic therapeutic classification (ATC) </li> | |||
<li>Substance name code (SNK) </li> | |||
<li>Substance name code with route of administration (SKK)</li> | |||
</ul> | |||
| | | | ||
| Regel 375: | Regel 375: | ||
|0..1 | |0..1 | ||
|The relative amount of this ingredient in this product. | |The relative amount of this ingredient in this product. | ||
Calculation of Concentration = Ingredient Amount | Calculation of Concentration = Ingredient Amount ÷ Product Amount. | ||
This could be a concentration if the medication is dissolved in liquid, for example. | This could be a concentration if the medication is dissolved in liquid, for example. | ||
| Regel 433: | Regel 433: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.13 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.13 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 442: | Regel 443: | ||
|Anatomic Therapeutic Classification (ATC) | |Anatomic Therapeutic Classification (ATC) | ||
|2.16.840.1.113883.6.73 | |2.16.840.1.113883.6.73 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodeGPKCodelist=== | === IngredientCodeGPKCodelist=== | ||
| Regel 448: | Regel 453: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.9 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.9 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 457: | Regel 463: | ||
|G-Standaard Generieke Product Kode (GPK) | |G-Standaard Generieke Product Kode (GPK) | ||
|2.16.840.1.113883.2.4.4.1 | |2.16.840.1.113883.2.4.4.1 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodeGTINCodelist=== | === IngredientCodeGTINCodelist=== | ||
| Regel 463: | Regel 473: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.16 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.16 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 472: | Regel 483: | ||
|Global Trade Item Number (GTIN) | |Global Trade Item Number (GTIN) | ||
|1.3.160 | |1.3.160 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodeHPKCodelist=== | === IngredientCodeHPKCodelist=== | ||
| Regel 478: | Regel 493: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.10 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.10 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 487: | Regel 503: | ||
|G-Standaard Handels Product Kode (HPK) | |G-Standaard Handels Product Kode (HPK) | ||
|2.16.840.1.113883.2.4.4.7 | |2.16.840.1.113883.2.4.4.7 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodePRKCodelist=== | === IngredientCodePRKCodelist=== | ||
| Regel 493: | Regel 513: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.11 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.11 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 502: | Regel 523: | ||
|G-Standaard Voorschrijfproducten (PRK) | |G-Standaard Voorschrijfproducten (PRK) | ||
|2.16.840.1.113883.2.4.4.10 | |2.16.840.1.113883.2.4.4.10 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodeSNKCodelist=== | === IngredientCodeSNKCodelist=== | ||
| Regel 508: | Regel 533: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.14 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.14 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 517: | Regel 543: | ||
|G-standaard Stofnaamcode (SNK) | |G-standaard Stofnaamcode (SNK) | ||
|2.16.840.1.113883.2.4.4.1.750 | |2.16.840.1.113883.2.4.4.1.750 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodeSSKCodelist=== | === IngredientCodeSSKCodelist=== | ||
| Regel 523: | Regel 553: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.15 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.15 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 532: | Regel 563: | ||
|G-standaard Stofnaamcode i.c.m. toedieningsweg (SSK) | |G-standaard Stofnaamcode i.c.m. toedieningsweg (SSK) | ||
|2.16.840.1.113883.2.4.4.1.725 | |2.16.840.1.113883.2.4.4.1.725 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== IngredientCodeZICodelist=== | === IngredientCodeZICodelist=== | ||
| Regel 538: | Regel 573: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.12 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.12 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 547: | Regel 583: | ||
|G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | |G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | ||
|2.16.840.1.113883.2.4.4.8 | |2.16.840.1.113883.2.4.4.8 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== PharmaceuticalFormCodelist=== | === PharmaceuticalFormCodelist=== | ||
| Regel 553: | Regel 593: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.8 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.8 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 562: | Regel 603: | ||
|G-Standaard Farmaceutische vormen | |G-Standaard Farmaceutische vormen | ||
|2.16.840.1.113883.2.4.4.11 | |2.16.840.1.113883.2.4.4.11 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== ProductCodeATCCodelist=== | === ProductCodeATCCodelist=== | ||
| Regel 568: | Regel 613: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.7 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.7 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 577: | Regel 623: | ||
|Anatomic Therapeutic Classification (ATC) | |Anatomic Therapeutic Classification (ATC) | ||
|2.16.840.1.113883.6.73 | |2.16.840.1.113883.6.73 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== ProductCodeGPKCodelist=== | === ProductCodeGPKCodelist=== | ||
| Regel 583: | Regel 633: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.6 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.6 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 592: | Regel 643: | ||
|G-Standaard Generieke Product Kode (GPK) | |G-Standaard Generieke Product Kode (GPK) | ||
|2.16.840.1.113883.2.4.4.1 | |2.16.840.1.113883.2.4.4.1 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== ProductCodeGTINCodelist=== | === ProductCodeGTINCodelist=== | ||
| Regel 598: | Regel 653: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.2 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.2 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 607: | Regel 663: | ||
|Global Trade Item Number (GTIN) | |Global Trade Item Number (GTIN) | ||
|1.3.160 | |1.3.160 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== ProductCodeHPKCodelist=== | === ProductCodeHPKCodelist=== | ||
| Regel 613: | Regel 673: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.5 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.5 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 622: | Regel 683: | ||
|G-Standaard Handels Product Kode (HPK) | |G-Standaard Handels Product Kode (HPK) | ||
|2.16.840.1.113883.2.4.4.7 | |2.16.840.1.113883.2.4.4.7 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== ProductCodePRKCodelist=== | === ProductCodePRKCodelist=== | ||
| Regel 628: | Regel 693: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.3 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.3 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 637: | Regel 703: | ||
|G-Standaard Voorschrijfproducten (PRK) | |G-Standaard Voorschrijfproducten (PRK) | ||
|2.16.840.1.113883.2.4.4.10 | |2.16.840.1.113883.2.4.4.10 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
=== ProductCodeZICodelist=== | === ProductCodeZICodelist=== | ||
| Regel 643: | Regel 713: | ||
|Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.1 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.1 | ||
|Binding<!--vsBindingTag-->: Required | |Binding<!--vsBindingTag-->: Required | ||
|Status<!--vsStatusTag-->: Active | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
| Regel 652: | Regel 723: | ||
|G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | |G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | ||
|2.16.840.1.113883.2.4.4.8 | |2.16.840.1.113883.2.4.4.8 | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |||
|colspan="3"| | |||
|-style="vertical-align:top; background-color: #EBF5FB;" | |||
|colspan="3"|Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset.<!--vsIncludeOTH--> | |||
|} | |} | ||
| Regel 665: | Regel 740: | ||
*[[DispenseRequest-v4.0(2024EN)|DispenseRequest-v4.0]] | *[[DispenseRequest-v4.0(2024EN)|DispenseRequest-v4.0]] | ||
*[[MedicationAdministration2-v3.0(2024EN)|MedicationAdministration2-v3.0]] | *[[MedicationAdministration2-v3.0(2024EN)|MedicationAdministration2-v3.0]] | ||
*[[MedicationAgreement- | *[[MedicationAgreement-v4.0(2024EN)|MedicationAgreement-v4.0]] | ||
*[[MedicationDispense-v3.0.1(2024EN)|MedicationDispense-v3.0.1]] | *[[MedicationDispense-v3.0.1(2024EN)|MedicationDispense-v3.0.1]] | ||
*[[MedicationUse2-v3.0(2024EN)|MedicationUse2-v3.0]] | *[[MedicationUse2-v3.0(2024EN)|MedicationUse2-v3.0]] | ||
*[[Vaccination-v6.0.1(2024EN)|Vaccination-v6.0.1]] | *[[Vaccination-v6.0.1(2024EN)|Vaccination-v6.0.1]] | ||
==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | ==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | ||
To exchange information based on health and care information models, additional, more technical specifications are required. | To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | ||
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:<!--ftReferenceIntro--> | Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:<!--ftReferenceIntro--> | ||
<ul> | <ul> | ||
<li> | <li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment<!--ftArtDecorReference--> {{ArtDecorLinks|2024|9.7}}</li> | ||
<li> | <li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR<!--ftSimplifierReference--> {{SimplefierLinks|2024|FarmaceutischProduct}}</li> | ||
</ul> | </ul> | ||
==Downloads<!--ftDownloadTitle-->== | ==Downloads<!--ftDownloadTitle-->== | ||
This information model is also available as [[Media:nl.zorg.part.PharmaceuticalProduct-v2.2.1(2024EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.part.PharmaceuticalProduct-v2.2.1(2024EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]]<!--ftDownloads--> | This information model is also available as [[Media:nl.zorg.part.PharmaceuticalProduct-v2.2.1(2024EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.part.PharmaceuticalProduct-v2.2.1(2024EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]]<!--ftDownloads--> | ||
==About this information<!--ftHeader2-->== | ==About this information<!--ftHeader2-->== | ||
The information in this wikipage is based on<!--ftInfoBase--> | The information in this wikipage is based on<!--ftInfoBase--> Release 2024 <BR> | ||
SNOMED CT and LOINC codes are based on: | SNOMED CT and LOINC codes are based on: | ||
<ul> | <ul> | ||
<li>SNOMED Clinical Terms versie: | <li>SNOMED Clinical Terms versie: 20250331 [R] (maart 2025-editie)</li> | ||
<li>LOINC version 2.77</li> | <li>LOINC version 2.77</li> | ||
</ul> | </ul> | ||
Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | ||
This page is generated on | This page is generated on 18/04/2025 17:57:36 with ZibExtraction v. 9.4.9209.40417<!--ftDate--> <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2024(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2024(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2024(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2024(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | ||
Huidige versie van 18 apr 2025 om 17:25
General information
Name: nl.zorg.part.PharmaceuticalProduct ![]()
Version: 2.2.1
HCIM Status:Final
Release: 2024
Release status: Published
Release date: 23-04-2025
Metadata
| DCM::CoderList | Projectgroep Medicatieproces |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Medicatieproces |
| DCM::CreationDate | 1-3-2017 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.7 |
| DCM::KeywordList | FarmaceutischProduct |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Architectuurgroep Registratie aan de Bron |
| DCM::Name | nl.zorg.part.FarmaceutischProduct |
| DCM::PublicationDate | 23-04-2025 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Medicatieproces & Architectuurgroep Registratie aan de Bron |
| DCM::RevisionDate | 13-09-2023 |
| DCM::Supersedes | nl.zorg.part.FarmaceutischProduct-v2.2 |
| DCM::Version | 2.2.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (04-09-2017)
Publicatieversie 2.0 (31-12-2017)
| ZIB-472 | Terminologiekoppeling klopt niet met beschrijving |
| ZIB-618 | Hernoemen Verstrekking naar Medicatieverstrekking |
Publicatieversie 2.1 (06-07-2019)
| ZIB-781 | aanpassing omschrijving 'Farmaceutische vorm' |
| ZIB-788 | Geen informatie |
| ZIB-795 | aanpassing voorbeeld 'Product (geneesmiddel)' |
| ZIB-796 | aanpassing definitie 'ProductCode (geneesmiddelcode)' |
| ZIB-797 | aanpassing definitie 'Product (geneesmiddel)' |
Publicatieversie 2.1.1 (31-01-2020)
| ZIB-789 | aanpassing voorbeeld en omschrijving 'Ingredientcode' (in zib FarmaceutischProduct) |
Publicatieversie 2.1.2 (01-09-2020)
| ZIB-911 | Correction English "Farmaceutical" |
Publicatieversie 2.1.3 (01-12-2021)
| ZIB-1452 | Geen informatie |
Publicatieversie 2.2 (10-06-2022)
| ZIB-1453 | Geen informatie |
| ZIB-1651 | Geen informatie |
| ZIB-1726 | Geen informatie |
Publicatieversie 2.2.1 (15-10-2023)
| ZIB-1743 | Geen informatie |
Concept
The prescribed substance is usually medication. However, medical aids and bandages can also be prescribed and supplied via the pharmacy. Food and blood products do not strictly belong in the medication category, but can be prescribed and supplied by a pharmacy as well.
A type of medication can be indicated with a single code. That code can be chosen from several possible coding systems (concretely: GPK, PRK, HPK or article numbers). Correct use of these codes in the software systems will sufficiently record the composition of the product used, making a complete product specification unnecessary.
In addition to a primary code, alternative codes from other coding systems can also be entered (so that the GPK can be sent along in the event that the patient was registered based on PRK, for example).
Entering multiple ingredients will enable you to display a compound product. If one of the composite parts is liquid, the dosage will be given in milliliters; otherwise it will be given in ‘units’.
In that case, the composition of the medication can be specified implicitly (with the use of a medication code) or explicitly, for example by listing the (active) ingredient(s) of the medication.
Magistral prescriptions can be entered as well. This can be done by means of the option listed above to enter coded ingredients and/or by entering the composition and preparation method as free text.
This is a partial information model
Purpose
The purpose of Product is to unambiguously describe the medication to be used.
Information Model
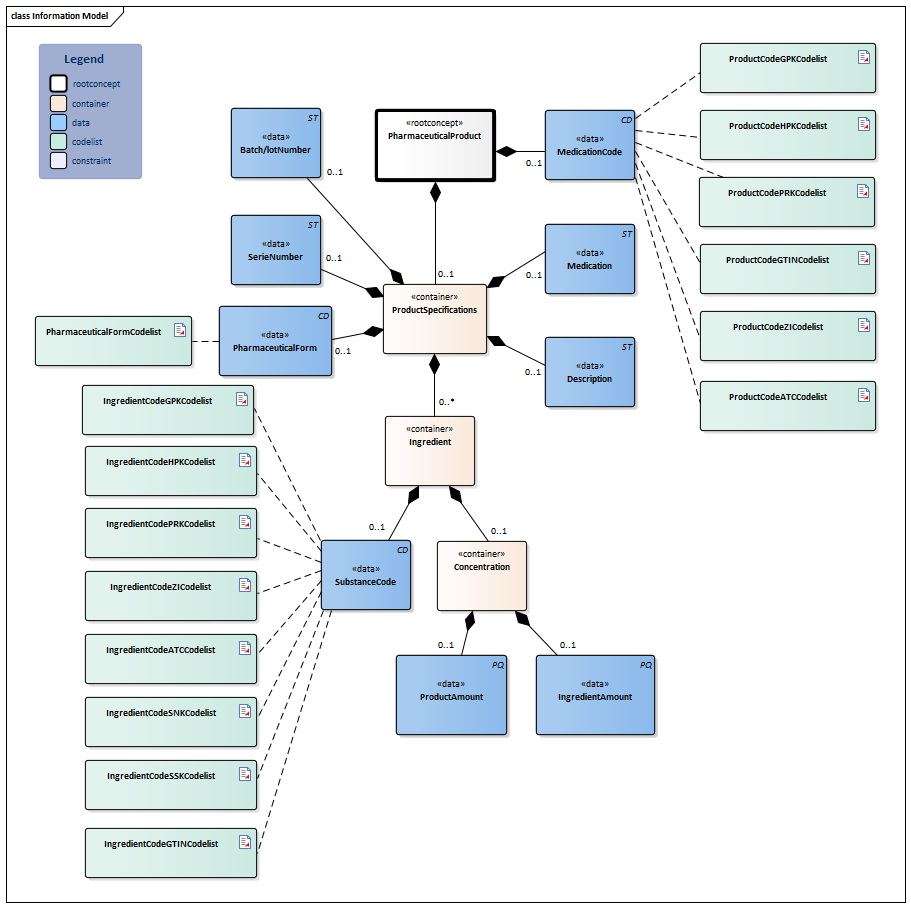
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||||||||||
| NL-CM:9.7.19926 | Root concept of the PharmaceuticalProduct partial information model. This root concept contains all data elements of the PharmaceuticalProduct partial information model. | ||||||||||||||||||||||
| NL-CM:9.7.19927 | 0..1 | Coding medication in the Netherlands is done on the basis of the G standard (issued by Z index), which is filled under the direction of KNMP.
The coded medication can be expressed as:
The GTIN enables identification of the product including its origin with a barcode. The ZI number represents the trade product and the package size. The HPK is the code for the trade product (with the brand name) as used per dose/per time the medication is taken (1 pill, 1 puff, 1ml). The PRK contains als GPK information supplemented with information needed to prescribe the right product. This level is intended to facilitate generic prescription of drugs. The GPK defines the pharmaceutical characteristics of a product: ingredients, strengths, units, pharmaceutical form and route of administration. The Substance Name Code (SNK) is the (active) compound. Further information about the G Standaard levels see https://www.z-index.nl . So-called 90.000.000 numbers are used in local IT systems. These numbers shall not be used for external communication. In this case only the description (not the code) may be included. This is in accordance with national agreements (Nictiz Standard). |
| ||||||||||||||||||||
| NL-CM:9.7.19928 | 0..1 | Container of the ProductSpecifications concept. This container contains all data elements of the ProductSpecifications concept.
Product specifications are required if the product code is not sufficient to ascertain the active substances and strength. |
|||||||||||||||||||||
| NL-CM:9.7.22279 | 0..1 | The batch/lot number provides a unique identification of the origin of information such as the production batch or machine number. The number is constructed according to the GS1 standard AI(10).( https://www.gs1.nl/sectorafspraken-over-standaarden/unieke-identificatie-en-productdata-gezondheidszorg/gs1-barcodes-2#application-identifiers-ais ) | |||||||||||||||||||||
| NL-CM:9.7.22280 | 0..1 | The serial number uniquely identifies the source of information of the dose itself. The number is constructed according to the GS1 standard AI(21). (<A title="Volg link" href="https://www.gs1.nl/sectorafspraken-over-standaarden/unieke-identificatie-en-productdata-gezondheidszorg/gs1-barcodes-2#application-identifiers-ais">https://www.gs1.nl/sectorafspraken-over-standaarden/unieke-identificatie-en-productdata-gezondheidszorg/gs1-barcodes-2#application-identifiers-ais</A>) | |||||||||||||||||||||
| NL-CM:9.7.19931 | 0..1 | The pharmaceutical form indicates the form of the medication. Examples include: tablet, suppository, infusion liquid, ointment. If the product has a GPK code in the G standard, the form will be known in the G standard. For products without a code (free text, preparation by the pharmacy), the means of administration can be entered. |
| ||||||||||||||||||||
| NL-CM:9.7.19929 | 0..1 | For medication which has no code, enter the complete name of the pharmaceutical product here. | |||||||||||||||||||||
| NL-CM:9.7.19784 | 0..1 | A textual description of the type of medication (including relevant properties of the composition and preparation method if possible), which is only used if no coded indication from the G Standard is available (historical data regarding magistral preparation which has been entered as free text instead of entering the substance codes). | |||||||||||||||||||||
| NL-CM:9.7.19932 | 0..* | Container of the Ingredient concept. This container contains all data elements of the Ingredient concept.
A product contains one or more active substances and excipients. These are usually determined by the product code. For medication prepared or compounded by the local pharmacy, each ingredient must be entered separately. The active substances play an important role, as they: a) determine the pharmacotherapeutic effect of the medication and b) serve as the basis for the indication of the strength of the medication (e.g. 200 mg). |
|||||||||||||||||||||
| NL-CM:9.7.19934 | 0..1 | Active substance or excipient.
Here, the same codes can be used as for the ProductCode (for dilutions and compounds in particular), but now, the SSK and SNK codes can also be used to indicate a substance (to list ingredients of local products prepared by the pharmacy).
|
|||||||||||||||||||||
| NL-CM:9.7.19933 | 0..1 | The relative amount of this ingredient in this product.
Calculation of Concentration = Ingredient Amount ÷ Product Amount. This could be a concentration if the medication is dissolved in liquid, for example. |
|||||||||||||||||||||
| NL-CM:9.7.22277 | 0..1 | The amount and unit of this ingredient. This is the numerator for the calculation of the concentration. The unit should be selected from the G Standard (Table 902). | |||||||||||||||||||||
| NL-CM:9.7.22278 | 0..1 | Amount of the product. This is the denominator for the calculation of the concentration. Optionally a translation to NHG table Gebruiksvoorschriften (Table 25) is also allowed. | |||||||||||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| Afgesproken geneesmiddel |
| Farmaceutischproduct |
| Lisinopril tablet 10mg |
| Methotrexaat injvlst 25mg/ml 0,6 ml |
References
1. ATC [Online] Beschikbaar op: https://www.whocc.no/atc/structure_and_principles// [Geraadpleegd: 13 september 2023].
Valuesets
IngredientCodeATCCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.13 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | Anatomic Therapeutic Classification (ATC) | 2.16.840.1.113883.6.73 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodeGPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.9 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Generieke Product Kode (GPK) | 2.16.840.1.113883.2.4.4.1 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodeGTINCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.16 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | Global Trade Item Number (GTIN) | 1.3.160 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodeHPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.10 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodePRKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.11 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Voorschrijfproducten (PRK) | 2.16.840.1.113883.2.4.4.10 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodeSNKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.14 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Stofnaamcode (SNK) | 2.16.840.1.113883.2.4.4.1.750 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodeSSKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.15 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-standaard Stofnaamcode i.c.m. toedieningsweg (SSK) | 2.16.840.1.113883.2.4.4.1.725 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
IngredientCodeZICodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.12 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | 2.16.840.1.113883.2.4.4.8 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
PharmaceuticalFormCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.8 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Farmaceutische vormen | 2.16.840.1.113883.2.4.4.11 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
ProductCodeATCCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.7 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | Anatomic Therapeutic Classification (ATC) | 2.16.840.1.113883.6.73 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
ProductCodeGPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.6 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Generieke Product Kode (GPK) | 2.16.840.1.113883.2.4.4.1 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
ProductCodeGTINCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.2 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | Global Trade Item Number (GTIN) | 1.3.160 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
ProductCodeHPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.5 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
ProductCodePRKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.3 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Voorschrijfproducten (PRK) | 2.16.840.1.113883.2.4.4.10 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
ProductCodeZICodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.1 | Binding: Required | Status: Active |
| Conceptname | Codesystem name | Codesystem OID |
| All values | G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | 2.16.840.1.113883.2.4.4.8 |
| Value Other (OTH) from codesystem NullFlavor (OID: 2.16.840.1.113883.5.1008) is allowed in this valueset. | ||
This information model in other releases
- Release 2017, (Version 2.0)
- Prerelease 2018-2, (Version 2.0)
- Prerelease 2019-2, (Version 2.1.1)
- Release 2020, (Version 2.1.2)
- Prerelease 2021-2, (Version 2.1.3)
- Prerelease 2022-1, (Version 2.2)
- Prerelease 2023-1, (Version 2.2.1)
- Prerelease 2024-1, (Versie 2.2.1)
Information model references
This information model refers to
- --
This information model is used in
- AdministrationAgreement-v3.0
- DispenseRequest-v4.0
- MedicationAdministration2-v3.0
- MedicationAgreement-v4.0
- MedicationDispense-v3.0.1
- MedicationUse2-v3.0
- Vaccination-v6.0.1
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release 2024
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms versie: 20250331 [R] (maart 2025-editie)
- LOINC version 2.77
Conditions for use are located on the mainpage ![]()
This page is generated on 18/04/2025 17:57:36 with ZibExtraction v. 9.4.9209.40417