LaboratoryTestResult-v4.0(2017EN)
General information
Name: nl.zorg.LaboratoryTestResult ![]()
Version: 4.0
HCIM Status:Final
Release: 2017
Release status: Prepublished
Release date: 04-09-2017
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 7-6-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.13.1 |
| DCM::KeywordList | laboratorium uitslag, lab, laboratorium bepaling |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.LaboratoriumUitslag |
| DCM::PublicationDate | 04-09-2017 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 04-09-2017 |
| DCM::Superseeds | nl.zorg.OverdrachtLaboratoriumUitslag-v3.0 |
| DCM::Version | 4.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-238 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept TestNaam opsplitsen. |
| ZIB-239 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet SNOMED - eLab valueset van concept Testmethode opsplitsen. |
| ZIB-240 | In de klinische bouwsteen OverdrachtLabUitslag kwam de tagged value DCM::ValueSet van concept LaboratoriumTest niet overeen met de naam van de gekoppelde waardenlijst ResultNormalcyStatus Valueset (HL7). |
| ZIB-241 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept Onderzoek opsplitsen. |
| ZIB-242 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatStatus niet overeen met de tagged value van het concept. |
| ZIB-243 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatType niet overeen met de tagged value van het concept. |
| ZIB-244 | Tagged values van concept Onderzoek van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-245 | Tagged values van concept Testmethode van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-246 | Tagged values van concept TestNaam van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
| ZIB-361 | Naamgeving concept Opmerking aangepast |
| ZIB-367 | Opschonen ResultaatVlaggenCodelijst |
| ZIB-370 | ResultaatStatusCodelijst en TekstUitslagCodelijst codes aanpassen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 1.2.1 (22-05-2015)
| ZIB-392 | De ResultaatTypeCodelijst heeft geen "OID: " aanduiding in de onderliggende codelijst. |
Publicatieversie 1.2.2 (16-07-2015)
| ZIB-420 | Vervallen SNOMED CT code in ResultaatTypeCodelijst |
Publicatieversie 3.0 (01-05-2016)
| ZIB-423 | Verkeerd bron-codestelsel gekoppeld aan de 'TestStatusCodelijst'. |
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
Publicatieversie 4.0 (04-09-2017)
| ZIB-479 | Monster herkomst ontbreekt |
| ZIB-549 | De Engelse naam van de bouwsteen en het rootconcept zijn niet correct |
| ZIB-564 | Aanpassing/harmonisatie Engelse conceptnamen |
| ZIB-576 | Aanpassen bouwstenen die nog de prefix overdracht hebben, zodat de prefix kan vervallen. |
| ZIB-481 | Ambiguïteit in interpretatie van ResultaatStatus |
| ZIB-577 | Toevoegen SNOMED CT concept in ResultaatTypeCodelijst |
Concept
A laboratory result describes the result of a laboratory analysis.
These are specimen-oriented tests as performed in laboratories such as Clinical Chemistry, Serology, Microbiology, etc.
In addition to the results of tests with a singular result, this concept can also contain the results of more complex tests with multiple results or a ‘panel’.
Purpose
Laboratory tests are done for the purpose of diagnosing and preventing disease and follow-up on the effects of treatment.
Evidence Base
There are two information models for recording laboratory test results: TextResult and LaboratoryTestResult.
In the case of laboratory test results, it is difficult to clearly indicate exactly when to use this information model and when to use the TextResult information model.
In general, laboratory tests resulting in a value (7.1 mmol/L), ordinal number (++ from series to ++++) or a quantitative result (Low) are recorded using this information model. The TextResult information model is better suited for textual results that are more descriptive in nature and which are longer than just a few words. Both types of tests occur in almost all laboratories.
The applicability of the aforementioned information models is not determined by the kind of lab but by the kind of result.
Information Model
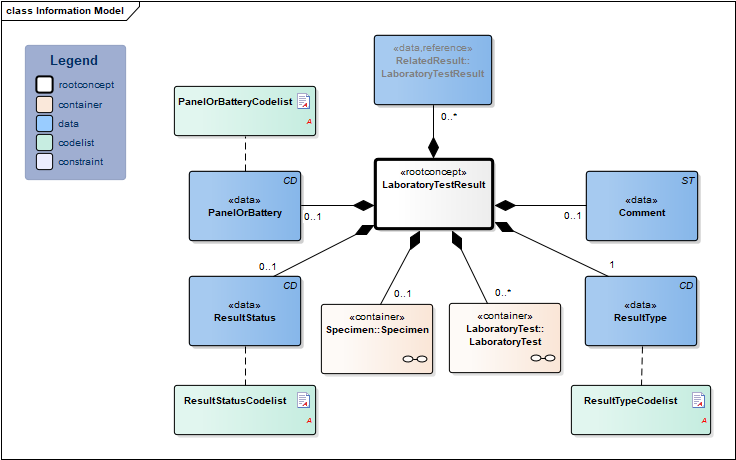
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | ||||||||
| NL-CM:13.1.1 | Root concept of the LaboratoryTestResult information model. This root concept contains all data elements of the LaboratoryTestResult information model. | |||||||||||||
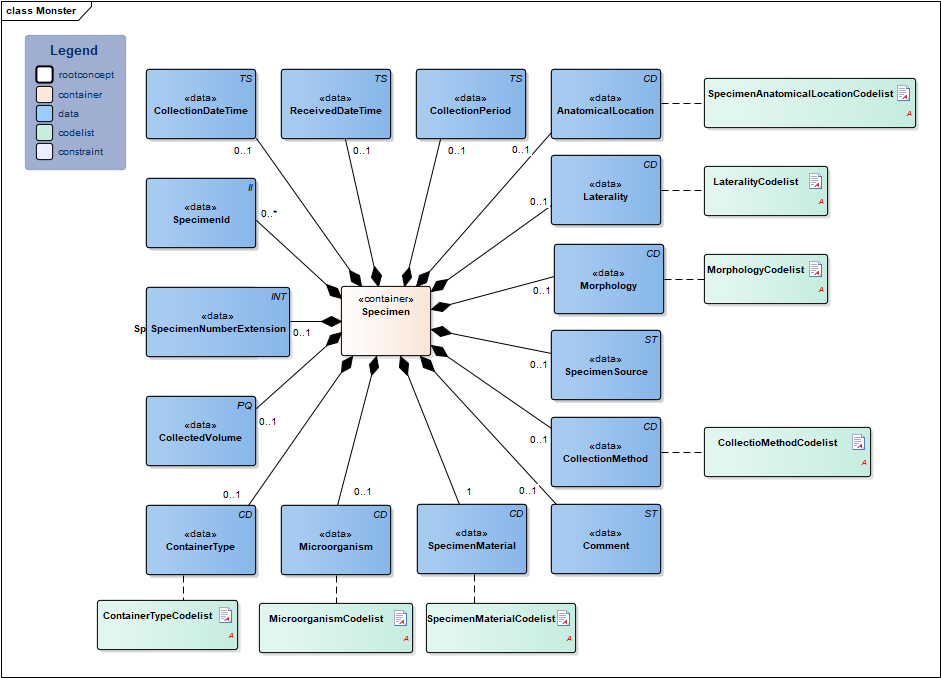
| NL-CM:13.1.2 | 0..1 | Container of the Specimen concept. This container contains all data elements of the Specimen concept. |
|
|||||||||||
| NL-CM:13.1.15 | 0..* | Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | ||||||||||||
| NL-CM:13.1.20 | 0..1 | The specimen number extension is used when the collected material is distributed from the original tube or container across multiple tubes. In combination with the specimen Id the extension yield a unique identification of the tube or container | ||||||||||||
| NL-CM:13.1.21 | 0..1 | Container type describes the envelope in which the material is collected or sent. Examples include blood tubes, transport container, possibly including culture medium. |
| |||||||||||
| NL-CM:13.1.16 | 1 | SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level.
This is in line with the agreements made in the IHE/Nictiz program e-Lab. If the test is carried out on derived material (such as plasma), this element will still contain the material drawn (in this case, blood). In this case, the LOINC code will generally refer to plasma. |
|
| ||||||||||
| NL-CM:13.1.22 | 0..1 | In particular in microbiological determinations the subject of the test is an isolate of certain microorganism rather then a material. This concept provides the ability to capture information about this microorganism. |
| |||||||||||
| NL-CM:13.1.23 | 0..1 | Total volume of the collected material. If it is necessary to determine the absolute amount of a particular substance in the collected material, the volume thereof must be given. | ||||||||||||
| NL-CM:13.1.24 | 0..1 | If the material has not been collected at a single point in time but over a certain period, this period can be captured in this concept. An example is 24 hour urine. | ||||||||||||
| NL-CM:13.1.17 | 0..1 | Time at which the material was collected. |
|
|||||||||||
| NL-CM:13.1.25 | 0..1 | Date and time that the material is handed over at the laboratory or specimen collection center. This is the issue with material that is collected by the patient himself. | ||||||||||||
| NL-CM:13.1.18 | 0..1 | If relevant for the results, the method of obtaining the specimen can be entered as well. |
|
| ||||||||||
| NL-CM:13.1.26 | 0..1 | Anatomic location where the material is collected, e.g. elbow |
|
| ||||||||||
| NL-CM:13.1.27 | 0..1 | Laterality adds information about body side to the anatomic location, e.g. left |
|
| ||||||||||
| NL-CM:13.1.28 | 0..1 | Morphology describes morphological abnormalities of the anatomical location where the material is taken, for example wound, ulcer. |
|
| ||||||||||
| NL-CM:13.1.29 | 0..1 | If the material is not collected directly from the patient but comes from a patient-related object, e.g. a cathetertip, this source of material can be recorded here. |
|
|||||||||||
| NL-CM:13.1.19 | 0..1 | Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. |
|
|||||||||||
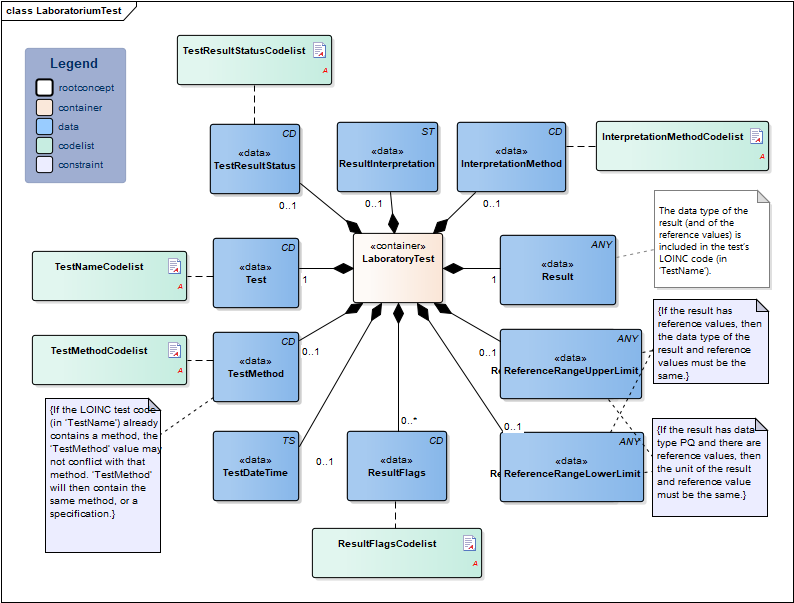
| NL-CM:13.1.3 | 0..* | Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | ||||||||||||
| NL-CM:13.1.8 | 1 | The name and code of the executed test. |
| |||||||||||
| NL-CM:13.1.9 | 0..1 | The test method used to obtain the result. |
|
| ||||||||||
| NL-CM:13.1.13 | 0..1 | The date and if possible the time at which the test was carried out. | ||||||||||||
| NL-CM:13.1.10 | 1 | The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | ||||||||||||
| NL-CM:13.1.31 | 0..1 | The status of the test result of this test. If the laboratory test is an panel/cluster, the overall status is given in the status of the panel/cluster. |
| |||||||||||
| NL-CM:13.1.11 | 0..1 | The upper reference limit for the patient of the value measured in the test. | ||||||||||||
| NL-CM:13.1.12 | 0..1 | The lower reference limit for the patient of the value measured with the test. | ||||||||||||
| NL-CM:13.1.30 | 0..1 | The method used to determine interpretation flags. An example of this is EUCAST, for determining clinical breakpoints in microbiological susceptibility tests |
| |||||||||||
| NL-CM:13.1.14 | 0..* | Attention codes indicating whether the result is above or below certain reference values or interpreting the result otherwise.(Resistent) |
|
| ||||||||||
| NL-CM:13.1.32 | 0..1 | Comment of the laboratory specialist regarding the interpretation of the results |
|
|||||||||||
| NL-CM:13.1.4 | 0..1 | For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. |
| |||||||||||
| NL-CM:13.1.6 | 0..1 | The status of the laboratory test result .If the laboratory test is an panel/cluster, this status reflects the status of the whole panel/cluster. If the status item per subtest is used too, this status must be in accordance with these status values. |
| |||||||||||
| NL-CM:13.1.5 | 0..1 | Comments, such as a textual interpretation or advice accompanying the result, for example. |
|
|||||||||||
| NL-CM:13.1.7 | 1 | The type of result defines the laboratory specialty under which the test is categorized. |
| |||||||||||
| NL-CM:13.1.33 | 0..* | Reference to related tests, e.g. paired tests or sequential tests like gram staining and microbiological cultures |
| |||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
Test | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 12-06-2012 09:00 | Natrium | 12-06-2012 13:15 | 138 mmol/l | 136 mmol/l | 146 mmol/l | |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
Test | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 23-05-2012 08:08 | Chloride | 23-05-2012 12:00 | 109 mmol/l | 99 mmol/l | 108 mmol/l | Boven referentie- waarde |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
Test | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Virologie | Definitief | Bloed | 16-01-2012 08:00 | Hepatitis A IgM | 16-01-2012 10:12 | Negatief | |||
References
1. Nederlandse Vereniging voor Medische Microbiologie (2010) ELab en EvT. [Online] Beschikbaar op: http://www.nvmm.nl/ict/vereniging/werkgroepen_commissies/elab-en-evt [Geraadpleegd: 23 juli 2014].
Valuesets
CollectioMethodCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <17636008 | specimen collection | | SNOMED CT | 2.16.840.1.113883.6.96 |
ContainerTypeCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.9 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 434711009 |Specimen container (physical object)| | SNOMED CT | 2.16.840.1.113883.6.96 |
InterpretationMethodCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.14 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| EUCAST | tbd | SNOMED CT | 2.16.840.1.113883.6.96 | EUCAST |
LateralityCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.12 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Left | 7771000 | SNOMED CT | 2.16.840.1.113883.6.96 | Links |
| Right | 24028007 | SNOMED CT | 2.16.840.1.113883.6.96 | Rechts |
| Right and left | 51440002 | SNOMED CT | 2.16.840.1.113883.6.96 | Rechts en links |
MicroorganismCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.10 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: ^2581000146104 |simpele referentieset voor micro-organismen (foundation metadata concept)| | SNOMED CT | 2.16.840.1.113883.6.96 |
MorphologyCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.13 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 49755003 |Morphologically abnormal structure| | SNOMED CT | 2.16.840.1.113883.6.96 |
PanelOrBatteryCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
ResultFlagsCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Above reference range | 281302008 | SNOMED CT | 2.16.840.1.113883.6.96 | Boven referentiewaarde |
| Below reference range | 281300000 | SNOMED CT | 2.16.840.1.113883.6.96 | Onder referentiewaarde |
| Intermediate | 11896004 | SNOMED CT | 2.16.840.1.113883.6.96 | Intermediair |
| Resistant | 30714006 | SNOMED CT | 2.16.840.1.113883.6.96 | Resistent |
| Susceptible | 131196009 | SNOMED CT | 2.16.840.1.113883.6.96 | Sensitief |
ResultStatusCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
ResultTypeCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Hematology | 252275004 | SNOMED CT | 2.16.840.1.113883.6.96 | Hematologie |
| Chemistry | 275711006 | SNOMED CT | 2.16.840.1.113883.6.96 | Klinische chemie |
| Serology | 68793005 | SNOMED CT | 2.16.840.1.113883.6.96 | Serologie/ immunologie |
| Virology | 395124008 | SNOMED CT | 2.16.840.1.113883.6.96 | Virologie |
| Toxicology | 314076009 | SNOMED CT | 2.16.840.1.113883.6.96 | Toxicologie |
| Microbiology | 19851009 | SNOMED CT | 2.16.840.1.113883.6.96 | Microbiologie |
| Molecular genetics | 405825005 | SNOMED CT | 2.16.840.1.113883.6.96 | Moleculaire genetica |
SpecimenAnatomicalLocationCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.11 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 442083009 |Anatomical or acquired body structure| | SNOMED CT | 2.16.840.1.113883.6.96 |
SpecimenMaterialCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 105590001 |substantie| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestMethodCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 272394005 |Technique (qualifier value)| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestNameCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
TestResultStatusCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.15 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
This information model in other releases
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2017 #1
Conditions for use are located on the mainpage ![]()
This page is generated on 30/11/2017 13:37:14