MedicalDevice-v4.0(2022EN)
General information
Name: nl.zorg.MedicalDevice ![]()
Version: 4.0
HCIM Status:Final
Release: 2022
Release status: Prepublished
Release date: 15-10-2023
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 2-1-2013 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.10.1 |
| DCM::KeywordList | medisch hulpmiddel, implantaat |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.MedischHulpmiddel |
| DCM::PublicationDate | 15-10-2023 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 17-07-2023 |
| DCM::Supersedes | nl.zorg.MedischHulpmiddel-v3.5 |
| DCM::Version | 4.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013) -
Publicatieversie 1.1 (01-07-2013)
Issue summaries niet beschikbaarBevat: ZIB-11.
Publicatieversie 1.2 (01-04-2015) Bevat: ZIB-83, ZIB-88, ZIB-110, ZIB-249, ZIB-250, ZIB-251, ZIB-252, ZIB-308, ZIB-327, ZIB-353.
Incl. algemene wijzigingsverzoeken: ZIB-94, ZIB-154, ZIB-200, ZIB-201, ZIB-309, ZIB-324, ZIB-326.
Publicatieversie 3.0 (01-05-2016) Bevat: ZIB-453.
Publicatieversie 3.1 (04-09-2017) Bevat: ZIB-457, ZIB-461, ZIB-517, ZIB-522, ZIB-547, ZIB-549, ZIB-564,. ZIB-568, ZIB-573, ZIB-574, ZIB-578, ZIB-585.
Publicatieversie 3.1.1 (01-10-2018) Bevat: ZIB-673.
Publicatieversie 3.2 (26-02-2019) Bevat: ZIB-680.
Publicatieversie 3.3 (06-07-2019) Bevat: ZIB-734.
Publicatieversie 3.3.1 (01-09-2020) Bevat: ZIB-1116, ZIB-1135, ZIB-1120.
Publicatieversie 3.4 (01-12-2021) Bevat: ZIB-1279, ZIB-1534.
Publicatieversie 3.5 (10-06-2022) Bevat: ZIB-1535.
Publicatieversie 4.0 (15-10-2023) Bevat: ZIB-1536, ZIB-1778, ZIB-1974.
Concept
Medical devices are any internally implanted and external devices and/or aids used by the patient (in the past) to reduce the effects of functional limitations in organ systems or to facilitate the treatment of a disease.
Purpose
Data on medical devices is recorded for several reasons. Knowledge of the presence of these implants enables tracing and taking the aid or device into account in diagnostic or therapeutic procedures, care and transport.
Examples include:
- Consequences for transportation, toilet use, etc., in the case of a wheelchair;
- A pacemaker can be of medical importance, but also has consequences for planning radiological exams.
Evidence Base
Recording data on medically complex devices such as pacemakers is not yet common in EPD systems in the Netherlands, but is sometimes lacking: a letter from a specialist for example often does not include information on which type of pacemaker the patient has (and from which manufacturer).
The Dutch Ministry of Health, Welfare and Sport (VWS) will pass legislation for a national basic register of implants. Every healthcare center will have to supply a UDI (Unique Device Identification, with a link to GTIN) and a UPI (Unique Patient Identification) to the basic register. This will prevent situations in which a large group of patients have an aid or implant in which problems have been detected that cannot be traced.
Information Model
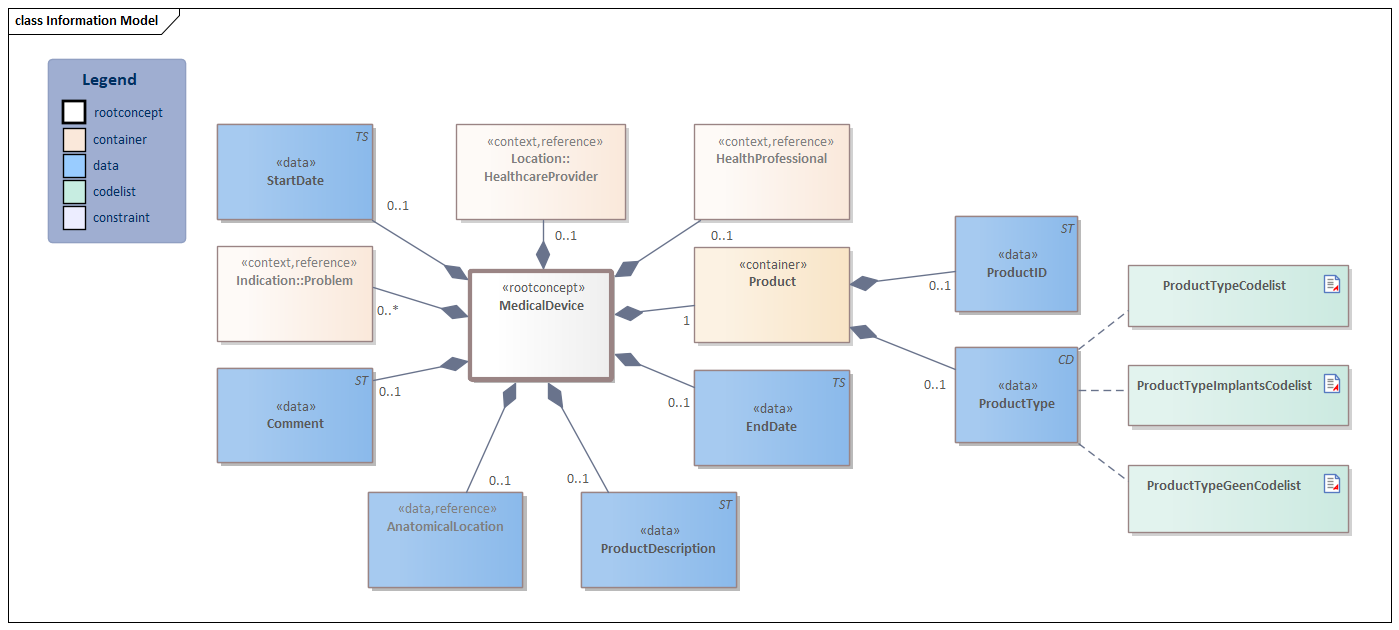
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||||
| NL-CM:10.1.1 | Root concept of the MedicalDevice information model. This root concept contains all data elements of the MedicalDevice information model. |
|
|||||||||||||||
| NL-CM:10.1.2 | 1 | The medical device (internally or externally). |
|
||||||||||||||
| NL-CM:10.1.3 | 0..1 | The code of the type of product. |
| ||||||||||||||
| NL-CM:10.1.16 | 0..1 | Globally unique identification of the product, for example the serial number or a UDI (unique device identifier). For some products, the law requires the use of a UDI. Commonly used coding systems are HIBC and GS1/GTIN.
A UDI often contains more information than just an ID, but also, for example, an expiration date. If a UDI is used, the entire code can be included as text in ProductID, so that no important information is lost. |
|||||||||||||||
| NL-CM:10.1.13 | 0..1 | Textual description of the product. | |||||||||||||||
| NL-CM:10.1.15 | 0..1 | Patient’s anatomical location of the medical device used. |
|
| |||||||||||||
| NL-CM:10.1.7 | 0..* | The medical reason for use of the medical device. |
| ||||||||||||||
| NL-CM:10.1.11 | 0..1 | The start date of the first use or implant of the medical device. A ‘vague’ date, such as only the year, is permitted. | |||||||||||||||
| NL-CM:10.1.14 | 0..1 | The end date of the last use or explant of the medical device. A ‘vague’ date, such as only the year, is permitted. | |||||||||||||||
| NL-CM:10.1.10 | 0..1 | Comment about use or information on the medical device used. |
|
||||||||||||||
| NL-CM:10.1.8 | 0..1 | The healthcare provider where use of the medical device was initiated or where the aid was implanted. |
| ||||||||||||||
| NL-CM:10.1.9 | 0..1 | The health professional involved in the indication for use of the medical device implant. |
| ||||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
Voorbeeld file fout: Unable to find the specified file. : nl.zorg.Zib of versie niet gevonden-v4.0(NL)_Voorbeeld.docx
References
1. Kamerbrief over het voorstel voor een register van implantaten. [Online] Beschikbaar op: http://www.rijksoverheid.nl/documenten-en-publicaties/kamerstukken/2012/11/20/kamerbrief-over-het-voorstel-voor-een-register-van-implantaten.html [Geraadpleegd: 15 september 2014].
Valuesets
ProductTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.1 | Binding: Required |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <260787004|Physical object| | SNOMED CT | 2.16.840.1.113883.6.96 |
ProductTypeGeenCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.7 | Binding: Required |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Geen | 0 | Hulpmiddelen gehoor | 2.16.840.1.113883.2.4.3.11.22.211 | geen gehoorapparaat |
| Geen | 0 | eCare codes | 2.16.840.1.113883.2.4.3.11.22.218 | geen visuele hulpmiddelen |
Note: Intended to be used with VisualFunction and HearingFunction.
ProductTypeImplantsCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.6 | Binding: Required |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: ^52801000146101|Dutch implant registry simple reference set| | SNOMED CT | 2.16.840.1.113883.6.96 |
This information model in other releases
- Release 2015, (Version 1.2)
- Release 2016, (Version 3.0)
- Release 2017, (Version 3.1)
- Prerelease 2018-2, (Version 3.2)
- Prerelease 2019-2, (Version 3.3)
- Release 2020, (Version 3.3.1)
- Prerelease 2021-2, (Version 3.4)
- Prerelease 2023-1, (Version 4.0)
- Prerelease 2024-1, (Versie 4.0)
- Release 2024, (Version 5.0)
Information model references
This information model refers to
This information model is used in
- AbilityToPerformMouthcareActivities-v3.1
- BladderFunction-v4.0
- BowelFunction-v4.0
- FeedingTubeSystem-v3.4
- FunctionalOrMentalStatus-v3.2.1
- HearingFunction-v3.3
- Infusion-v3.4
- Mobility-v3.3.1
- NursingIntervention-v4.0
- Procedure-v5.4
- Respiration-v3.2
- Stoma-v3.4
- VisualFunction-v3.3
- Wound-v3.4
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Pre-release 2022-1
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms versie: 20230930 [R] (september 2023-editie)
- LOINC version 2.76
Conditions for use are located on the mainpage ![]()
This page is generated on 27/03/2024 11:10:24 with ZibExtraction v. 9.3.8851.20230