LaboratoryTestResultForTransfer-v3.0(2016EN)
General information
Name: nl.zorg.LaboratoryTestResultForTransfer ![]()
Version: 3.0
HCIM Status:Final
Release: 2016
Release status: Published
Release date: 1-5-2016
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 7-6-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.13.1 |
| DCM::KeywordList | laboratorium uitslag, lab, laboratorium bepaling |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.OverdrachtLaboratoriumUitslag |
| DCM::PublicationDate | 1-5-2016 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 25-8-2015 |
| DCM::Superseeds | nl.nfu.OverdrachtLaboratoriumUitslag-v1.2.2 |
| DCM::Version | 3.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-238 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept TestNaam opsplitsen. |
| ZIB-239 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet SNOMED - eLab valueset van concept Testmethode opsplitsen. |
| ZIB-240 | In de klinische bouwsteen OverdrachtLabUitslag kwam de tagged value DCM::ValueSet van concept LaboratoriumTest niet overeen met de naam van de gekoppelde waardenlijst ResultNormalcyStatus Valueset (HL7). |
| ZIB-241 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept Onderzoek opsplitsen. |
| ZIB-242 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatStatus niet overeen met de tagged value van het concept. |
| ZIB-243 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatType niet overeen met de tagged value van het concept. |
| ZIB-244 | Tagged values van concept Onderzoek van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-245 | Tagged values van concept Testmethode van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-246 | Tagged values van concept TestNaam van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
| ZIB-361 | Naamgeving concept Opmerking aangepast |
| ZIB-367 | Opschonen ResultaatVlaggenCodelijst |
| ZIB-370 | ResultaatStatusCodelijst en TekstUitslagCodelijst codes aanpassen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 1.2.1 (22-05-2015)
| ZIB-392 | De ResultaatTypeCodelijst heeft geen "OID: " aanduiding in de onderliggende codelijst. |
Publicatieversie 1.2.2 (16-07-2015)
| ZIB-420 | Vervallen SNOMED CT code in ResultaatTypeCodelijst |
Publicatieversie 3.0 (01-05-2016)
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
.
Concept
A laboratory result describes the result of a laboratory analysis.
In addition to the results of tests with a singular result, the results of more complex tests with multiple results or a ‘panel’ can also be recorded.
Purpose
Laboratory tests are done for the purpose of diagnosing and preventing disease and follow-up on the effects of treatment.
Evidence Base
There are two information models for recording laboratory test results: TextResultTransfer and LaboratoryResultTransfer.
In the case of laboratory test results, it is difficult to clearly indicate exactly when to use this information model and when to use the TextResultTransfer information model.
In general, laboratory tests resulting in a value (7.1 mmol/L), ordinal number (++ from series to ++++) or a quantitative result (Low) are recorded using this information model. The TextResultTransfer information model is better suited for textual results that are more descriptive in nature and which are longer than just a few words. Both types of tests occur in almost all laboratories.
The applicability of the aforementioned information models is not determined by the kind of lab but by the kind of result.
In developing the information model, the definitions were used from the data set and coding choices from the IHE/Nictiz e-Lab program.
The now determined information model is a subset of the e-Lab data set, provided that the detailing that is less relevant to the general transfer use case was left out. If this information is required, it can be entered in the comments field.
Information Model
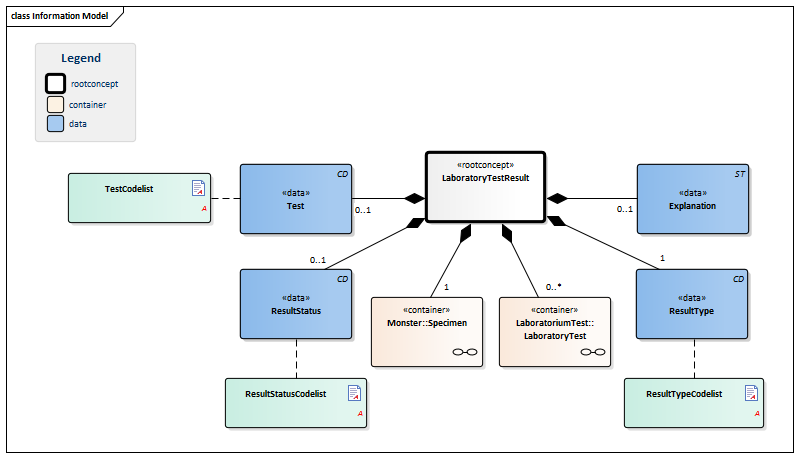
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:13.1.1 | Root concept of the LaboratoryTestResultTransfer information model. This root concept contains all data elements of the Laboratory TestResultTransfer information model. | ||||||||||||
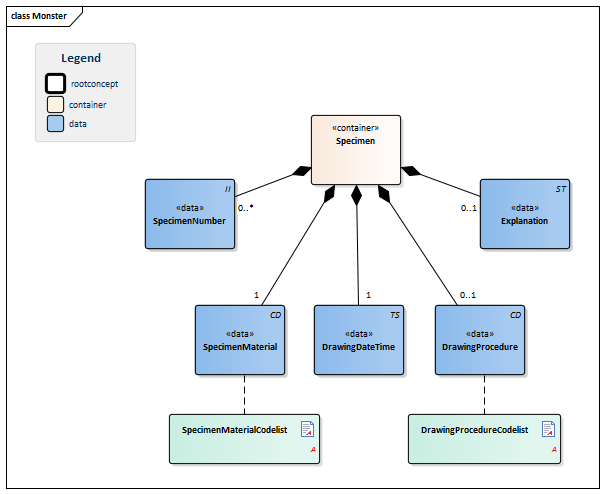
| NL-CM:13.1.2 | 1 | Container of the Specimen concept. This container contains all data elements of the Specimen concept. | |||||||||||
| NL-CM:13.1.15 | 0..* | Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | |||||||||||
| NL-CM:13.1.16 | 1 | SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level.
This is in line with the agreements made in the IHE/Nictiz program e-Lab. If the test is carried out on derived material (such as plasma), this element will still contain the material drawn (in this case, blood). In this case, the LOINC code will generally refer to plasma. |
| ||||||||||
| NL-CM:13.1.17 | 1 | Time at which the material was drawn. |
|
||||||||||
| NL-CM:13.1.18 | 0..1 | If relevant for the results, the method of obtaining the specimen can be entered as well. |
| ||||||||||
| NL-CM:13.1.19 | 0..1 | Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. |
|
||||||||||
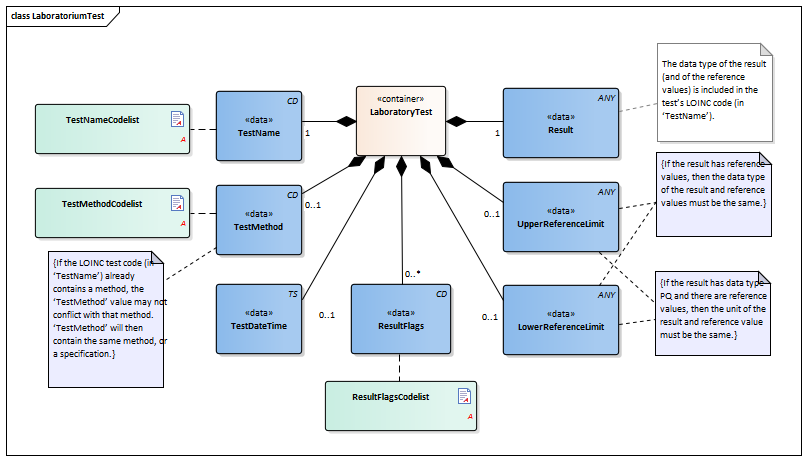
| NL-CM:13.1.3 | 0..* | Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | |||||||||||
| NL-CM:13.1.8 | 1 | The TestName is the name of the executed test. |
| ||||||||||
| NL-CM:13.1.9 | 0..1 | The test method used to obtain the result. |
| ||||||||||
| NL-CM:13.1.13 | 0..1 | The date and if possible the time at which the test was carried out. | |||||||||||
| NL-CM:13.1.10 | 1 | The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | |||||||||||
| NL-CM:13.1.11 | 0..1 | The upper reference limit for the patient of the value measured in the test. | |||||||||||
| NL-CM:13.1.12 | 0..1 | The lower reference limit for the patient of the value measured with the test. | |||||||||||
| NL-CM:13.1.14 | 0..* | Attention codes indicating whether the result is above or below certain reference values. |
| ||||||||||
| NL-CM:13.1.4 | 0..1 | For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. |
| ||||||||||
| NL-CM:13.1.6 | 0..1 | The status of the laboratory test result. |
| ||||||||||
| NL-CM:13.1.5 | 0..1 | Comments, such as a textual interpretation or advice accompanying the result, for example. |
|
||||||||||
| NL-CM:13.1.7 | 1 | The type of result defines the laboratory specialty under which the test is categorized. |
| ||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestNaam | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Resultaat Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 12-06-2012 09:00 | Natrium | 12-06-2012 13:15 | 138 mmol/l | 136 mmol/l | 146 mmol/l | |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestNaam | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Resultaat Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 23-05-2012 08:08 | Chloride | 23-05-2012 12:00 | 109 mmol/l | 99 mmol/l | 108 mmol/l | Boven referentie- waarde |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestNaam | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Resultaat Vlaggen | ||
| Virologie | Definitief | Bloed | 16-01-2012 08:00 | Hepatitis A IgM | 16-01-2012 10:12 | Negatief | |||
References
1. Nederlandse Vereniging voor Medische Microbiologie (2010) ELab en EvT. [Online] Beschikbaar op: http://www.nvmm.nl/ict/vereniging/werkgroepen_commissies/elab-en-evt [Geraadpleegd: 23 juli 2014].
Valuesets
DrawingProcedureCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <17636008|specimen collection| | SNOMED CT | 2.16.840.1.113883.6.96 |
ResultFlagsCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| High | H | ObservationInterpretation | 2.16.840.1.113883.5.83 | Boven referentiewaarde |
| Low | L | ObservationInterpretation | 2.16.840.1.113883.5.83 | Onder referentiewaarde |
| Intermediate | I | ObservationInterpretation | 2.16.840.1.113883.5.83 | Variabel |
| Resistant | R | ObservationInterpretation | 2.16.840.1.113883.5.83 | Resistent |
| Susceptible | S | ObservationInterpretation | 2.16.840.1.113883.5.83 | Sensitief |
ResultStatusCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
ResultTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Hematology | 252275004 | SNOMED CT | 2.16.840.1.113883.6.96 | Hematologie |
| Chemistry | 275711006 | SNOMED CT | 2.16.840.1.113883.6.96 | Klinische chemie |
| Serology | 68793005 | SNOMED CT | 2.16.840.1.113883.6.96 | Serologie/ immunologie |
| Virology | 395124008 | SNOMED CT | 2.16.840.1.113883.6.96 | Virologie |
| Toxicology | 314076009 | SNOMED CT | 2.16.840.1.113883.6.96 | Toxicologie |
| Microbiology | 19851009 | SNOMED CT | 2.16.840.1.113883.6.96 | Microbiologie |
SpecimenMaterialCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <123038009|specimen| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
TestMethodCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | SNOMED CT | 2.16.840.1.113883.6.96 |
TestNameCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
This information model in other releases
- Release 2015, (Version 1.2.2)
- Release 2017, (Version 4.1)
- Prerelease 2018-2, (Version 4.3)
- Prerelease 2019-2, (Version 4.5)
- Release 2020, (Version 4.6)
- Prerelease 2021-2, (Version 5.0)
- Prerelease 2022-1, (Version 5.1)
- Prerelease 2023-1, (Version 6.0)
- Prerelease 2024-1, (Versie 7.0)
- Release 2024, (Version 7.1)
Information model references
This information model refers to
- --
This information model is used in
- --
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release summer 2016
Conditions for use are located on the mainpage ![]()
This page is generated on 21/12/2018 15:40:38 with ZibExtraction v. 3.0.6929.24609