Medicatieafspraak-v1.0.1(2018EN)
General information
Name: nl.zorg.Medicatieafspraak ![]()
Version: 1.0.1
HCIM Status:Final
Release: 2018
Release status: Prepublished
Release date: 01-10-2018
Metadata
| DCM::CoderList | Projectgroep Medicatieproces |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Medicatieproces |
| DCM::CreationDate | 1-2-2017 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.6 |
| DCM::KeywordList | Medicatie, Afspraak |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.Medicatieafspraak |
| DCM::PublicationDate | 01-10-2018 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Medicatieproces & Architectuurgroep Registratie aan de Bron |
| DCM::RevisionDate | 31-12-2017 |
| DCM::Superseeds | nl.zorg.Medicatieafspraak-v1.0 |
| DCM::Version | 1.0.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (04-09-2017)
Publicatieversie 1.0.1 (31-12-2017)
| ZIB-618 | Hernoemen Verstrekking naar Medicatieverstrekking |
| ZIB-643 | Kleine tekstuele verbeteringen |
Concept
A medication agreement is a prescriber’s proposal for a patient to use medication. An agreement to discontinue the use of medication is also a medication agreement.
Purpose
The goal of the medication agreement is to provide insight into the agreements reached between the prescriber and the patient on the use of medication.
Information Model
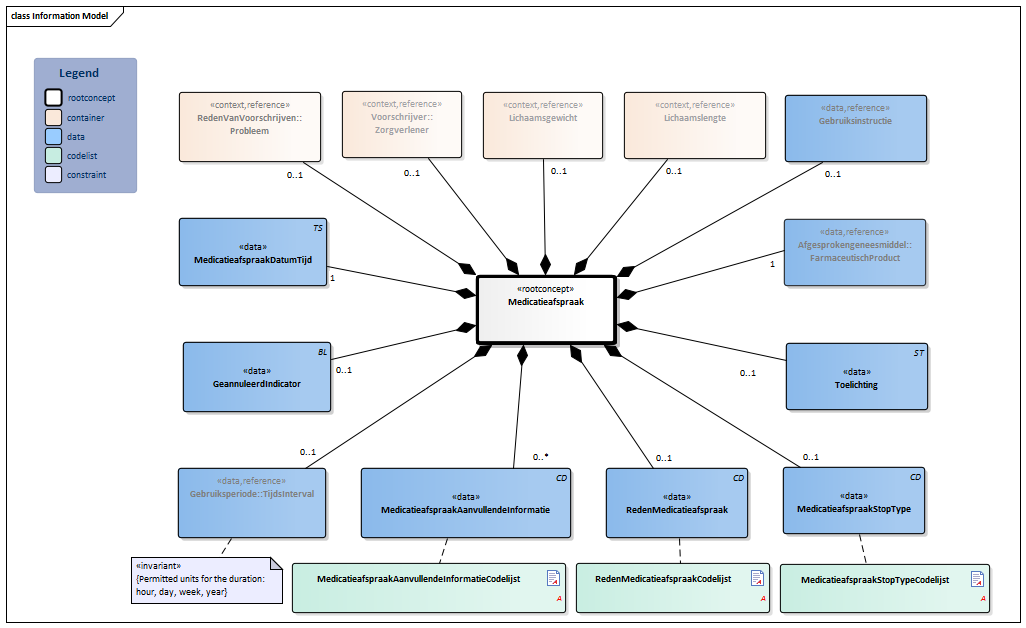
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:9.6.9580 | Root concept of the MedicationAgreement information model. This root concept contains all data elements of the MedicationAgreement information model. | ||||||||||||
| NL-CM:9.6.1030 | 0..1 | The health professional that entered the medication agreement with the patient. |
| ||||||||||
| NL-CM:9.6.23133 | 0..1 | The medical reason for the prescription or for use of the medication. This can be used to enter a medical indication which was the direct cause for prescription or for use of the medication in question.
It can concern every type of problem (or condition) of the patient, almost all diagnoses, complaints or symptoms. Please note: The BST401T file of the G standard contains a “special reference” to indicate that “exchange of the reason for prescription is essential”. |
| ||||||||||
| NL-CM:9.6.23028 | 0..1 | Weight the dose is based on, relevant to this agreement. |
| ||||||||||
| NL-CM:9.6.23023 | 0..1 | Height the dose is based on, relevant to this agreement. |
| ||||||||||
| NL-CM:9.6.19925 | 1 | The medicine agreed upon to be used. |
| ||||||||||
| NL-CM:9.6.23240 | 0..1 | Instructions for the use of the medication, e.g. dose and route of administration. |
| ||||||||||
| NL-CM:9.6.19757 | 1 | The time at which the agreement was made.
Appointment date + time are required (order of the appointments must be clear in cases with multiple appointments on one day) |
|||||||||||
| NL-CM:9.6.19936 | 0..1 | Start date: This is the time at which the agreement was to take effect (or took effect or will take effect). This is the time at which the instructions for use in this agreement start. In the case of an agreement to discontinue use, this is the start date of the original medication agreement. The end date indicates from when the medication is to be discontinued.
Duration: The intended duration of use. E.g. 5 days or 8 weeks. It is not allowed to indicate the duration in months, because different months have a variable duration in days. End date: The time at which the period of use ends (or ended or will end). In the case of an agreement to discontinue use, this is the time at which the medication is to be discontinued. To avoid confusion between 'to' and 'up to', the submission of time is always mandatory for the end date. With medication for an indefinite period only a start date is indicated. |
| ||||||||||
| NL-CM:9.6.23033 | 0..1 | In the event of an error correction of the medication agreement, this indicator is to be put on for the incorrect agreement. | |||||||||||
| NL-CM:9.6.19954 | 0..1 | Stop type, the manner in which this medication is discontinued (temporary or definitive). |
| ||||||||||
| NL-CM:9.6.22094 | 0..1 | Reason for this agreement. This can be the reason to start, change or stop the medication treatment. |
| ||||||||||
| NL-CM:9.6.23283 | 0..* | Additional information includes details on the structure of the agreement made that are relevant for pharmacovigilance and fulfillment by the pharmacist. This can be used e.g. to indicate that there was a conscious decision to deviate from the norm or that the agreement is to be structured in a certain way.
See also the Instructions section for more information about use of the element. |
| ||||||||||
| NL-CM:9.6.22273 | 0..1 | Comments regarding to the medication agreement. For example: in consultation with the medical specialist. |
|
||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| MedicatieAfspraak DatumTijd | Gebruiksperiode | Afgesproken geneesmiddel | Voorschrijver | Geannuleerd Indicator | MedicatieAfspraak Stoptype | MedicatieAfspraak.Reden | ||
| Ingangsdatum | Einddatum | Duur | FarmaceutischProduct | Zorgverlener | ||||
| Naamgegevens | ||||||||
| 18-9-2016 18:00:00 | 08-09-16 | 18-09-16 | Lisinopril tablet 10mg | D. Bakker | Nee | Definitief | Geen of onvoldoende effect | |
| 6-3-2016 9:12:30 | 06-03-2016 | Methotrexaat injvlst 25mg/ml 0,6 ml | R. Jansen | Nee | Starten medicamenteuze behandeling | |||
| RedenVan Voorschrijven | Gebruiksinstructie | |||
| Probleem | Omschrijving | Doseerinstructie | ||
| Doseerduur | Dosering |Keerdosis | Toedieningsschema |Frequentie |Interval |Toedientijd |Weekdag |Dagdeel | ||
| Van 8-9-2017 tot 18-9-2017 1x per dag 1 stuk. Vanaf 18-9-2017 staken. | 1 stuk | 1x per dag | ||
| Reumatoïde Artritis | Vanaf 6 maart 2016 1x per week op maandag om 14uur 15 mg (=0,6 ml) | 15 mg (=0,6 ml) | 1x per week op maandag (14u) | |
Instructions
MedicationAgreementAdditionalInformation:
When choosing a medicine, you can deviate from what is expected or from what the standard is. For example, when the hospital uses a different formulary than the community pharmacy. For reasons of efficiency, for example, one gastric acid inhibitor has been chosen in the hospital: pantoprazole.
Upon admission, a patient with omeprazole is converted to pantoprazole for the duration of the stay. When discharged, the patient goes back to omeprazole.
It is clear that something can go wrong here and that the patient takes both omeprazole and pantoprazole when there is no intervention. In the hospital's medication agreement for pantoprazole a remark can be made about the deviation so that it is clear that pantoprazole is the substitute for omeprazole or that it should be used in addition to omeprazole.
Another example are the half strengths. The hospital sometimes stocks tablets with half the strength of the normal trade preparation (own production). Where the patient enters the hospital on 25 mg chlortalidone, half a tablet once a day, he receives 12.5 mg intramural chlortalidone, one tablet once a day. Then the nursing does not have to break tablets in this case. Here there is a risk that the patient will use the 25 mg again at home, but then a whole tablet at a time in stead of half a tablet. By means of an explanation in the medication agreement (Additional information) of the last chlortalidone 25 mg, it can be indicated whether this has been a intended increase.
Valuesets
MedicatieafspraakAanvullendeInformatieCodelijst
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.6.3 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Bewust hoge dosering | 1 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Bewust hoge dosering |
| Bewust lage dosering | 2 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Bewust lage dosering |
| Bewust afwijkende toedieningsweg | 3 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Bewust afwijkende toedieningsweg |
| Medische noodzaak | 4 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Medische noodzaak |
| Profylaxe | 5 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Profylaxe |
| Wijziging in GDS per direct | 6 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Wijziging in GDS per direct |
| Wijziging in GDS per rolwissel | 7 | Voorlopige G-std thesaurus 2050 | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.3.2050 | Wijziging in GDS per rolwissel |
MedicatieafspraakStopTypeCodelijst
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.6.1 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Tijdelijk | 1 | StopType | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.1 | Tijdelijke onderbreking van medicamenteuze behandeling (bijvoorbeeld tijdelijk stoppen gebruik vanwege operatie). |
| Definitief | 2 | StopType | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.1 | Het staken van een bestaande medicamenteuze behandeling. |
RedenMedicatieafspraakCodelijst
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.9.6.2 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Medication commenced | 266709005 | SNOMED CT | 2.16.840.1.113883.6.96 | Starten medicamenteuze behandeling |
| Administration of medication contraindicated | 438833006 | SNOMED CT | 2.16.840.1.113883.6.96 | Contra-indicatie |
| Medication interaction | 79899007 | SNOMED CT | 2.16.840.1.113883.6.96 | Interactie |
| Hypersensitivity condition | 473010000 | SNOMED CT | 2.16.840.1.113883.6.96 | Overgevoeligheid |
| Geen of onvoldoende effect | 5 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Geen of onvoldoende effect |
| Te sterk effect | 6 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Te sterk effect |
| (Mogelijke) bijwerking | 7 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | (Mogelijke) bijwerking |
| Toedieningsweg voldoet niet | 8 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Toedieningsweg voldoet niet |
| Indicatie vervallen | 9 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Indicatie vervallen |
| Beleidswijziging | 10 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Beleidswijziging |
| Admission to establishment | 305335007 | SNOMED CT | 2.16.840.1.113883.6.96 | Opname |
| Wens patiënt | 12 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Wens patiënt |
| Volgens afspraak | 13 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Volgens afspraak |
| Hervatten beleid vorige voorschrijver | 14 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Hervatten beleid vorige voorschrijver |
| Geplande procedure | 15 | Medicatieafspraak Reden | 2.16.840.1.113883.2.4.3.11.60.20.77.5.2.2 | Procedure waaronder ingreep, interferentie met gepland labonderzoek, etc. |
| Overig | OTH | NullFlavour | 2.16.840.1.113883.5.1008 | Overig |
This information model in other releases
- Release 2017, (Version 1.0.1)
- Prerelease 2019-2, (Version 1.1)
- Release 2020, (Version 1.2)
- Prerelease 2021-2, (Version 1.3)
- Prerelease 2022-1, (Version 2.0)
- Prerelease 2023-1, (Version 2.1)
- Prerelease 2024-1, (Versie 3.0)
- Release 2024, (Version 4.0)
Information model references
This information model refers to
- BodyHeight-v3.1
- BodyWeight-v3.1
- HealthProfessional-v3.2
- InstructionsForUse-v1.1.1
- PharmaceuticalProduct-v2.0
- Problem-v4.1.1
- TimeInterval-v1.0
This information model is used in
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2018 #1
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms version: 20180731 [R] (July 2018 Release)
- LOINC version 2.64
Conditions for use are located on the mainpage ![]()
This page is generated on 23/12/2018 00:47:45 with ZibExtraction v. 3.0.6929.24609