LaboratoryTestResult-v4.2(2018EN): verschil tussen versies
Nieuwe pagina aangemaakt met '<!-- Hieronder wordt een transclude page aangeroepen --> {{Versions-2.16.840.1.113883.2.4.3.11.60.40.3.13.1(EN)|1|LaboratoryTestResult-v4.2(2018EN)}} <!-- Tot hie...' |
Geen bewerkingssamenvatting |
||
| Regel 231: | Regel 231: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
<imagemap> Bestand:LaboratoryTestResult-v4.2Model( | <imagemap> Bestand:LaboratoryTestResult-v4.2Model(2018EN).png | center | ||
rect | rect 623 84 735 154 [[LaboratoryTestResult-v4.2(2018EN)]] | ||
rect | rect 167 395 309 445 [[#ResultStatusCodelist]] | ||
rect | rect 167 96 309 146 [[#PanelOrBatteryCodelist]] | ||
rect | rect 626 185 738 255 [[#12848]] | ||
rect | rect 332 315 444 385 [[#12891]] | ||
rect | rect 611 396 751 446 [[#ResultTypeCodelist]] | ||
rect | rect 401 179 519 249 [[#12851]] | ||
rect | rect 459 315 571 385 [[#12870]] | ||
rect | rect 182 181 294 251 [[#12847]] | ||
rect | rect 625 286 737 356 [[#12844]] | ||
rect | rect 182 286 294 356 [[#12846]] | ||
rect | rect 321 43 460 113 [[HealthProfessional-v3.2(2018EN)]] | ||
rect | rect 472 43 611 113 [[HealthcareProvider-v3.2(2018EN)]] | ||
desc none | desc none | ||
</imagemap> | </imagemap> | ||
<imagemap> Bestand:LaboratoriumTest-v4.2Model( | <imagemap> Bestand:LaboratoriumTest-v4.2Model(2018EN).png | center | ||
rect | rect 187 43 342 93 [[#TestResultStatusCodelist]] | ||
rect | rect 220 132 310 202 [[#12859]] | ||
rect | rect 606 129 779 179 [[#InterpretationMethodCodelist]] | ||
rect | rect 467 130 576 200 [[#12869]] | ||
rect | rect 347 130 448 200 [[#12874]] | ||
rect | rect 42 343 197 393 [[#TestMethodCodelist]] | ||
rect | rect 42 259 197 309 [[#TestNameCodelist]] | ||
rect | rect 322 536 492 586 [[#ResultFlagsCodelist]] | ||
rect | rect 510 337 626 407 [[#12872]] | ||
rect | rect 357 439 457 509 [[#12866]] | ||
rect | rect 363 241 453 311 [[#12870]] | ||
rect | rect 510 243 626 313 [[#12865]] | ||
rect | rect 226 340 312 410 [[#12857]] | ||
rect | rect 223 246 309 316 [[#12861]] | ||
rect | rect 510 440 626 510 [[#12863]] | ||
rect | rect 223 439 309 509 [[#12875]] | ||
desc none | desc none | ||
</imagemap> | </imagemap> | ||
<imagemap> Bestand:Monster-v4.2Model( | <imagemap> Bestand:Monster-v4.2Model(2018EN).png | center | ||
rect | rect 269 611 423 661 [[#MicroorganismCodelist]] | ||
rect | rect 107 608 248 658 [[#ContainerTypeCodelist]] | ||
rect | rect 714 86 926 136 [[#SpecimenAnatomicalLocationCodelist]] | ||
rect | rect 714 174 838 224 [[#LateralityCodelist]] | ||
rect | rect 714 262 838 312 [[#MorphologyCodelist]] | ||
rect | rect 291 77 401 147 [[#12882]] | ||
rect | rect 561 163 671 233 [[#12884]] | ||
rect | rect 561 337 671 407 [[#12880]] | ||
rect | rect 156 295 266 365 [[#12879]] | ||
rect | rect 156 513 266 583 [[#12885]] | ||
rect | rect 156 404 266 474 [[#12894]] | ||
rect | rect 291 513 401 583 [[#12876]] | ||
rect | rect 426 77 536 147 [[#12886]] | ||
rect | rect 559 77 669 147 [[#12892]] | ||
rect | rect 565 252 675 322 [[#12889]] | ||
rect | rect 436 611 586 661 [[#SpecimenMaterialCodelist]] | ||
rect | rect 714 435 881 485 [[#CollectionMethodCodelist]] | ||
rect | rect 351 294 441 364 [[#12891]] | ||
rect | rect 561 425 671 495 [[#12887]] | ||
rect | rect 427 512 537 582 [[#12896]] | ||
rect | rect 156 186 266 256 [[#12898]] | ||
rect | rect 561 512 671 582 [[#12881]] | ||
rect | rect 156 77 266 147 [[#12883]] | ||
desc none | desc none | ||
</imagemap> | </imagemap> | ||
| Regel 879: | Regel 879: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: < [https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=71388002 71388002]<noWiki>|</noWiki><span title = "Verrichting" >Procedure</span><noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=71388002 71388002]<noWiki>|</noWiki><span title = "Verrichting">Procedure</span><noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 894: | Regel 894: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: < [https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=434711009 434711009]<noWiki>|</noWiki>Specimen container<noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=434711009 434711009]<noWiki>|</noWiki>Specimen container<noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 930: | Regel 930: | ||
|Description<!--vsDescription--> | |Description<!--vsDescription--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Links" >Left</span> | |<span title = "Links">Left</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=7771000 7771000] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=7771000 7771000] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 936: | Regel 936: | ||
|Links | |Links | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Rechts" >Right</span> | |<span title = "Rechts">Right</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=24028007 24028007] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=24028007 24028007] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 942: | Regel 942: | ||
|Rechts | |Rechts | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Bilateraal" >Right and left</span> | |<span title = "Bilateraal">Right and left</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=51440002 51440002] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=51440002 51440002] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 959: | Regel 959: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: ^ [https://terminologie.nictiz.nl/terminology/snomed/viewRefset?id=2581000146104 2581000146104]<noWiki>|</noWiki><span title = "Referentieset voor micro-organismen" >Dutch microorganism simple reference set</span><noWiki>|</noWiki> | |SNOMED CT: ^[https://terminologie.nictiz.nl/terminology/snomed/viewRefset?id=2581000146104 2581000146104]<noWiki>|</noWiki><span title = "Referentieset voor micro-organismen">Dutch microorganism simple reference set</span><noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 974: | Regel 974: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: < [https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=49755003 49755003]<noWiki>|</noWiki><span title = "Morfologische afwijking" >Morphologically abnormal structure</span><noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=49755003 49755003]<noWiki>|</noWiki><span title = "Morfologische afwijking">Morphologically abnormal structure</span><noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 1.092: | Regel 1.092: | ||
|Description<!--vsDescription--> | |Description<!--vsDescription--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Hematologisch onderzoek" >Hematology test</span> | |<span title = "Hematologisch onderzoek">Hematology test</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=252275004 252275004] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=252275004 252275004] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 1.098: | Regel 1.098: | ||
|Hematologie | |Hematologie | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Serumtest" >Serum chemistry test</span> | |<span title = "Serumtest">Serum chemistry test</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=275711006 275711006] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=275711006 275711006] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 1.110: | Regel 1.110: | ||
|Serologie/ immunologie | |Serologie/ immunologie | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Virologisch onderzoek" >Viral studies</span> | |<span title = "Virologisch onderzoek">Viral studies</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=395124008 395124008] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=395124008 395124008] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 1.116: | Regel 1.116: | ||
|Virologie | |Virologie | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Toxicologische test" >Toxicology screening test</span> | |<span title = "Toxicologische test">Toxicology screening test</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=314076009 314076009] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=314076009 314076009] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 1.122: | Regel 1.122: | ||
|Toxicologie | |Toxicologie | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "Microbiologisch onderzoek" >Microbiology procedure</span> | |<span title = "Microbiologisch onderzoek">Microbiology procedure</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=19851009 19851009] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=19851009 19851009] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 1.145: | Regel 1.145: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: < [https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=442083009 442083009]<noWiki>|</noWiki><span title = "Anatomische of verworven lichaamsstructuur" >Anatomical or acquired body structure</span><noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=442083009 442083009]<noWiki>|</noWiki><span title = "Anatomische of verworven lichaamsstructuur">Anatomical or acquired body structure</span><noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 1.160: | Regel 1.160: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: < [https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=105590001 105590001]<noWiki>|</noWiki><span title = "Substantie" >Substance</span><noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=105590001 105590001]<noWiki>|</noWiki><span title = "Substantie">Substance</span><noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 1.175: | Regel 1.175: | ||
|Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: < [https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=272394005 272394005]<noWiki>|</noWiki>Technique<noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=272394005 272394005]<noWiki>|</noWiki>Technique<noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 1.247: | Regel 1.247: | ||
*[[HealthProfessional-v3.2(2018EN)|HealthProfessional-v3.2]] | *[[HealthProfessional-v3.2(2018EN)|HealthProfessional-v3.2]] | ||
*[[LaboratoryTestResult-v4.2(2018EN)|LaboratoryTestResult-v4.2]] | *[[LaboratoryTestResult-v4.2(2018EN)|LaboratoryTestResult-v4.2]] | ||
====This | ====This information model is used in<!--ftReferredBy-->==== | ||
*[[LaboratoryTestResult-v4.2(2018EN)|LaboratoryTestResult-v4.2]] | *[[LaboratoryTestResult-v4.2(2018EN)|LaboratoryTestResult-v4.2]] | ||
==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | ==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | ||
| Regel 1.266: | Regel 1.266: | ||
</ul> | </ul> | ||
Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | ||
This page is generated on | This page is generated on 23/12/2018 01:18:08 with ZibExtraction v. 3.0.6929.24609<!--ftDate--> <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2018(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2018(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2018(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2018(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | ||
Huidige versie van 24 dec 2018 om 11:57
General information
Name: nl.zorg.LaboratoryTestResult ![]()
Version: 4.2
HCIM Status:Final
Release: 2018
Release status: Prepublished
Release date: 01-10-2018
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 7-6-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.13.1 |
| DCM::KeywordList | laboratorium uitslag, lab, laboratorium bepaling |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.LaboratoriumUitslag |
| DCM::PublicationDate | 01-10-2018 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 31-12-2017 |
| DCM::Superseeds | nl.zorg.LaboratoriumUitslag-v4.1 |
| DCM::Version | 4.2 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-238 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept TestNaam opsplitsen. |
| ZIB-239 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet SNOMED - eLab valueset van concept Testmethode opsplitsen. |
| ZIB-240 | In de klinische bouwsteen OverdrachtLabUitslag kwam de tagged value DCM::ValueSet van concept LaboratoriumTest niet overeen met de naam van de gekoppelde waardenlijst ResultNormalcyStatus Valueset (HL7). |
| ZIB-241 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept Onderzoek opsplitsen. |
| ZIB-242 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatStatus niet overeen met de tagged value van het concept. |
| ZIB-243 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatType niet overeen met de tagged value van het concept. |
| ZIB-244 | Tagged values van concept Onderzoek van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-245 | Tagged values van concept Testmethode van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-246 | Tagged values van concept TestNaam van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
| ZIB-361 | Naamgeving concept Opmerking aangepast |
| ZIB-367 | Opschonen ResultaatVlaggenCodelijst |
| ZIB-370 | ResultaatStatusCodelijst en TekstUitslagCodelijst codes aanpassen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 1.2.1 (22-05-2015)
| ZIB-392 | De ResultaatTypeCodelijst heeft geen "OID: " aanduiding in de onderliggende codelijst. |
Publicatieversie 1.2.2 (16-07-2015)
| ZIB-420 | Vervallen SNOMED CT code in ResultaatTypeCodelijst |
Publicatieversie 3.0 (01-05-2016)
| ZIB-423 | Verkeerd bron-codestelsel gekoppeld aan de 'TestStatusCodelijst'. |
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
Publicatieversie 4.0 (04-09-2017)
| ZIB-479 | Monster herkomst ontbreekt |
| ZIB-549 | De Engelse naam van de bouwsteen en het rootconcept zijn niet correct |
| ZIB-564 | Aanpassing/harmonisatie Engelse conceptnamen |
| ZIB-576 | Aanpassen bouwstenen die nog de prefix overdracht hebben, zodat de prefix kan vervallen. |
| ZIB-481 | Ambiguïteit in interpretatie van ResultaatStatus |
| ZIB-577 | Toevoegen SNOMED CT concept in ResultaatTypeCodelijst |
Publicatieversie 4.1 (31-12-2017)
| ZIB-609 | Biopsie niet in AfnameprocedureCodelijst |
| ZIB-611 | cardinaliteit ResultaatType |
| ZIB-621 | Aanpassingen naar aanleiding van overleg met NHG |
| ZIB-645 | Diverse terminologiekoppelingen lijken niet te kloppen |
| ZIB-646 | Aanpassing SNOMED codes |
Publicatieversie 4.2 (01-10-2018)
| ZIB-649 | Toevoegen data-element UitgevoerdDoor |
| ZIB-676 | Typefout in "CollectioMethodCodelist" |
Concept
A laboratory result describes the result of a laboratory analysis.
These are specimen-oriented tests as performed in laboratories such as Clinical Chemistry, Serology, Microbiology, etc.
In addition to the results of tests with a singular result, this concept can also contain the results of more complex tests with multiple results or a ‘panel’.
Purpose
Laboratory tests are done for the purpose of diagnosing and preventing disease and follow-up on the effects of treatment.
Evidence Base
There are two information models for recording laboratory test results: TextResult and LaboratoryTestResult.
In the case of laboratory test results, it is difficult to clearly indicate exactly when to use this information model and when to use the TextResult information model.
In general, laboratory tests resulting in a value (7.1 mmol/L), ordinal number (++ from series to ++++) or a quantitative result (Low) are recorded using this information model. The TextResult information model is better suited for textual results that are more descriptive in nature and which are longer than just a few words. Both types of tests occur in almost all laboratories.
The applicability of the aforementioned information models is not determined by the kind of lab but by the kind of result.
Information Model
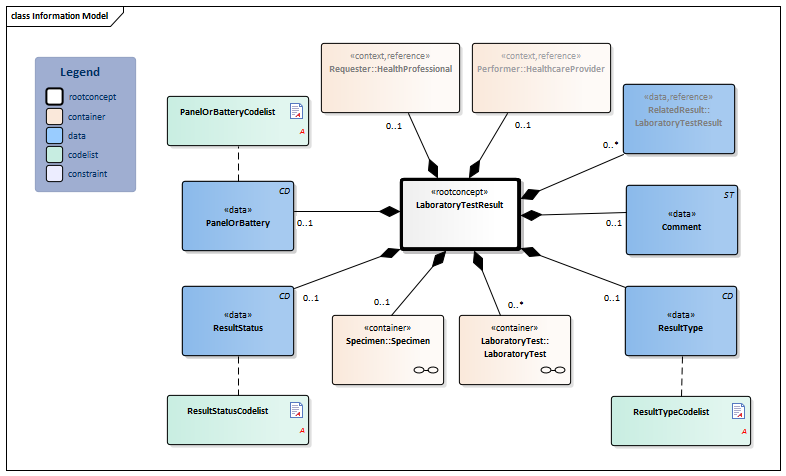
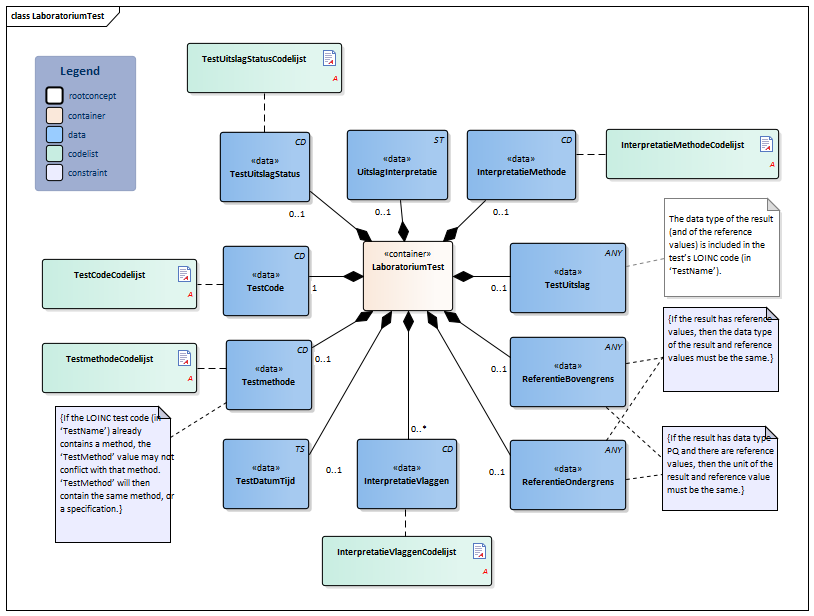
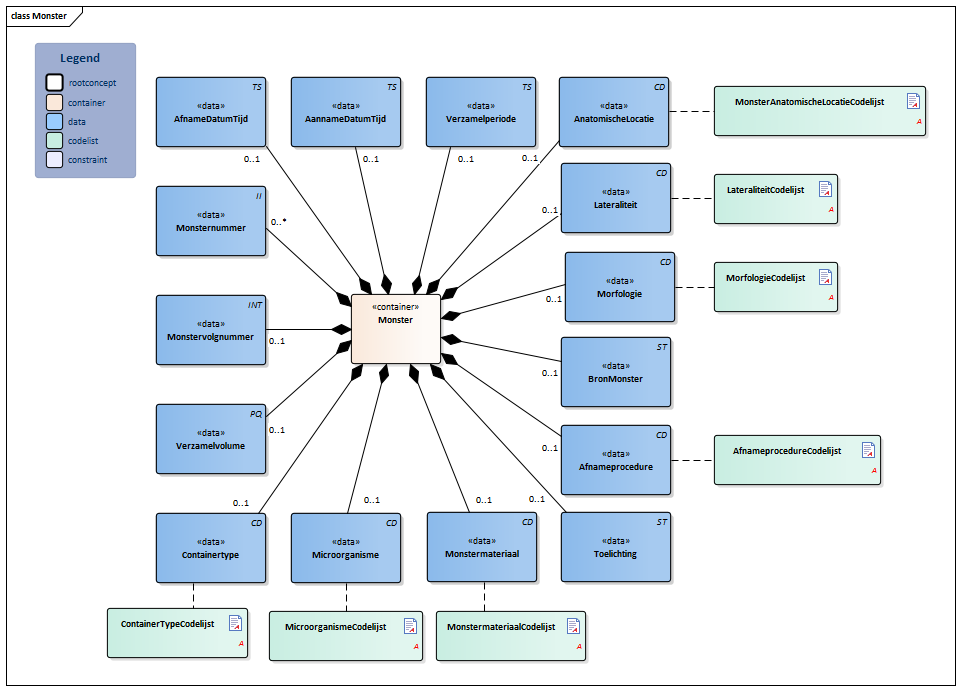
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | ||||||||
| NL-CM:13.1.1 | Root concept of the LaboratoryTestResult information model. This root concept contains all data elements of the LaboratoryTestResult information model. | |||||||||||||
| NL-CM:13.1.2 | 0..1 | Container of the Specimen concept. This container contains all data elements of the Specimen concept. |
|
|||||||||||
| NL-CM:13.1.15 | 0..* | Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | ||||||||||||
| NL-CM:13.1.20 | 0..1 | The specimen number extension is used when the collected material is distributed from the original tube or container across multiple tubes. In combination with the specimen Id the extension yield a unique identification of the tube or container | ||||||||||||
| NL-CM:13.1.21 | 0..1 | Container type describes the envelope in which the material is collected or sent. Examples include blood tubes, transport container, possibly including culture medium. |
| |||||||||||
| NL-CM:13.1.16 | 0..1 | SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level.
This is in line with the agreements made in the IHE/Nictiz program e-Lab. If the test is carried out on derived material (such as plasma), this element will still contain the material drawn (in this case, blood). In this case, the LOINC code will generally refer to plasma. |
|
| ||||||||||
| NL-CM:13.1.22 | 0..1 | In particular in microbiological determinations the subject of the test is an isolate of certain microorganism rather then a material. This concept provides the ability to capture information about this microorganism. |
| |||||||||||
| NL-CM:13.1.23 | 0..1 | Total volume of the collected material. If it is necessary to determine the absolute amount of a particular substance in the collected material, the volume thereof must be given. | ||||||||||||
| NL-CM:13.1.24 | 0..1 | If the material has not been collected at a single point in time but over a certain period, this period can be captured in this concept. An example is 24 hour urine. | ||||||||||||
| NL-CM:13.1.17 | 0..1 | Time at which the material was collected. |
|
|||||||||||
| NL-CM:13.1.25 | 0..1 | Date and time that the material is handed over at the laboratory or specimen collection center. This is the issue with material that is collected by the patient himself. | ||||||||||||
| NL-CM:13.1.18 | 0..1 | If relevant for the results, the method of obtaining the specimen can be entered as well. |
|
| ||||||||||
| NL-CM:13.1.26 | 0..1 | Anatomic location where the material is collected, e.g. elbow |
|
| ||||||||||
| NL-CM:13.1.27 | 0..1 | Laterality adds information about body side to the anatomic location, e.g. left |
|
| ||||||||||
| NL-CM:13.1.28 | 0..1 | Morphology describes morphological abnormalities of the anatomical location where the material is taken, for example wound, ulcer. |
|
| ||||||||||
| NL-CM:13.1.29 | 0..1 | If the material is not collected directly from the patient but comes from a patient-related object, e.g. a cathetertip, this source of material can be recorded here. |
|
|||||||||||
| NL-CM:13.1.19 | 0..1 | Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. |
|
|||||||||||
| NL-CM:13.1.3 | 0..* | Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | ||||||||||||
| NL-CM:13.1.8 | 1 | The name and code of the executed test. |
| |||||||||||
| NL-CM:13.1.9 | 0..1 | The test method used to obtain the result. |
|
| ||||||||||
| NL-CM:13.1.13 | 0..1 | The date and if possible the time at which the test was carried out. | ||||||||||||
| NL-CM:13.1.10 | 0..1 | The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | ||||||||||||
| NL-CM:13.1.31 | 0..1 | The status of the test result of this test. If the laboratory test is an panel/cluster, the overall status is given in the status of the panel/cluster. |
| |||||||||||
| NL-CM:13.1.11 | 0..1 | The upper reference limit for the patient of the value measured in the test. | ||||||||||||
| NL-CM:13.1.12 | 0..1 | The lower reference limit for the patient of the value measured with the test. | ||||||||||||
| NL-CM:13.1.30 | 0..1 | The method used to determine interpretation flags. An example of this is EUCAST, for determining clinical breakpoints in microbiological susceptibility tests |
| |||||||||||
| NL-CM:13.1.14 | 0..* | Attention codes indicating whether the result is above or below certain reference values or interpreting the result otherwise.(Resistent) |
|
| ||||||||||
| NL-CM:13.1.32 | 0..1 | Comment of the laboratory specialist regarding the interpretation of the results |
|
|||||||||||
| NL-CM:13.1.4 | 0..1 | For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. |
| |||||||||||
| NL-CM:13.1.6 | 0..1 | The status of the laboratory test result .If the laboratory test is an panel/cluster, this status reflects the status of the whole panel/cluster. If the status item per subtest is used too, this status must be in accordance with these status values. |
| |||||||||||
| NL-CM:13.1.5 | 0..1 | Comments, such as a textual interpretation or advice accompanying the result, for example. |
|
|||||||||||
| NL-CM:13.1.7 | 0..1 | The type of result defines the laboratory specialty under which the test is categorized. |
| |||||||||||
| NL-CM:13.1.33 | 0..* | Reference to related tests, e.g. paired tests or sequential tests like gram staining and microbiological cultures |
| |||||||||||
| NL-CM:13.1.34 | 0..1 | The healthcare provider and/or organization where or by whom the LaboratoryTestResult was requested. |
| |||||||||||
| NL-CM:13.1.35 | 0..1 | The healthcare provider and/or organization where or by whom the LaboratoryTestResult was performed. |
| |||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestCode | Test DatumTijd |
TestUitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 12-06-2012 09:00 | Natrium | 12-06-2012 13:15 | 138 mmol/l | 136 mmol/l | 146 mmol/l | |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestCode | Test DatumTijd |
TestUitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 23-05-2012 08:08 | Chloride | 23-05-2012 12:00 | 109 mmol/l | 99 mmol/l | 108 mmol/l | Boven referentie- waarde |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestCode | Test DatumTijd |
TestUitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Virologie | Definitief | Bloed | 16-01-2012 08:00 | Hepatitis A IgM | 16-01-2012 10:12 | Negatief | |||
References
1. Nederlandse Vereniging voor Medische Microbiologie (2010) ELab en EvT. [Online] Beschikbaar op: http://www.nvmm.nl/ict/vereniging/werkgroepen_commissies/elab-en-evt [Geraadpleegd: 23 juli 2014].
Valuesets
CollectionMethodCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <71388002|Procedure| | SNOMED CT | 2.16.840.1.113883.6.96 |
ContainerTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.9 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <434711009|Specimen container| | SNOMED CT | 2.16.840.1.113883.6.96 |
InterpretationMethodCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.14 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| EUCAST | tbd | SNOMED CT | 2.16.840.1.113883.6.96 | EUCAST |
LateralityCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.12 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Left | 7771000 | SNOMED CT | 2.16.840.1.113883.6.96 | Links |
| Right | 24028007 | SNOMED CT | 2.16.840.1.113883.6.96 | Rechts |
| Right and left | 51440002 | SNOMED CT | 2.16.840.1.113883.6.96 | Rechts en links |
MicroorganismCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.10 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: ^2581000146104|Dutch microorganism simple reference set| | SNOMED CT | 2.16.840.1.113883.6.96 |
MorphologyCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.13 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <49755003|Morphologically abnormal structure| | SNOMED CT | 2.16.840.1.113883.6.96 |
PanelOrBatteryCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
ResultFlagsCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Above reference range | 281302008 | SNOMED CT | 2.16.840.1.113883.6.96 | Boven referentiewaarde |
| Below reference range | 281300000 | SNOMED CT | 2.16.840.1.113883.6.96 | Onder referentiewaarde |
| Intermediate | 11896004 | SNOMED CT | 2.16.840.1.113883.6.96 | Intermediair |
| Resistant | 30714006 | SNOMED CT | 2.16.840.1.113883.6.96 | Resistent |
| Susceptible | 131196009 | SNOMED CT | 2.16.840.1.113883.6.96 | Sensitief |
ResultStatusCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
ResultTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Hematology test | 252275004 | SNOMED CT | 2.16.840.1.113883.6.96 | Hematologie |
| Serum chemistry test | 275711006 | SNOMED CT | 2.16.840.1.113883.6.96 | Klinische chemie |
| Serologic test | 68793005 | SNOMED CT | 2.16.840.1.113883.6.96 | Serologie/ immunologie |
| Viral studies | 395124008 | SNOMED CT | 2.16.840.1.113883.6.96 | Virologie |
| Toxicology screening test | 314076009 | SNOMED CT | 2.16.840.1.113883.6.96 | Toxicologie |
| Microbiology procedure | 19851009 | SNOMED CT | 2.16.840.1.113883.6.96 | Microbiologie |
| Molecular genetic test | 405825005 | SNOMED CT | 2.16.840.1.113883.6.96 | Moleculaire genetica |
SpecimenAnatomicalLocationCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.11 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <442083009|Anatomical or acquired body structure| | SNOMED CT | 2.16.840.1.113883.6.96 |
SpecimenMaterialCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <105590001|Substance| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestMethodCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <272394005|Technique| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestNameCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
TestResultStatusCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.15 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
This information model in other releases
- Release 2015, (Version 1.2.2)
- Release 2016, (Version 3.0)
- Release 2017, (Version 4.1)
- Prerelease 2019-2, (Version 4.5)
- Release 2020, (Version 4.6)
- Prerelease 2021-2, (Version 5.0)
- Prerelease 2022-1, (Version 5.1)
- Prerelease 2023-1, (Version 6.0)
- Prerelease 2024-1, (Versie 7.0)
- Release 2024, (Version 7.1)
Information model references
This information model refers to
This information model is used in
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2018 #1
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms version: 20180731 [R] (July 2018 Release)
- LOINC version 2.64
Conditions for use are located on the mainpage ![]()
This page is generated on 23/12/2018 01:18:08 with ZibExtraction v. 3.0.6929.24609