LaboratoryTestResult-v4.0(2017EN): verschil tussen versies
Geen bewerkingssamenvatting |
Copy of |
||
| (2 tussenliggende versies door dezelfde gebruiker niet weergegeven) | |||
| Regel 3: | Regel 3: | ||
Version: '''4.0''' <br> | Version: '''4.0''' <br> | ||
HCIM Status:Final<br> | HCIM Status:Final<br> | ||
Release: '''2017''' <br> | |||
Release status: Prepublished<br> | Release status: Prepublished<br> | ||
Release date: 04-09-2017 | |||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | ||
| Regel 199: | Regel 199: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
<imagemap> Bestand:LaboratoryTestResult-v4.0Model(EN).png | center | |||
[[Bestand: LaboratoriumTest-v4.0Model(EN).png | center]] | rect 374 35 492 105 [[LaboratoryTestResult-v4.0(2017EN)]] | ||
[[Bestand: Monster-v4.0Model(EN).png | center]] | rect 174 385 316 435 [[#ResultStatusCodelist]] | ||
rect 174 86 316 136 [[#PanelOrBatteryCodelist]] | |||
rect 586 171 698 241 [[#12848]] | |||
rect 321 304 433 374 [[#12891]] | |||
rect 571 385 711 435 [[#ResultTypeCodelist]] | |||
rect 375 159 493 229 [[#12851]] | |||
rect 449 303 561 373 [[#12870]] | |||
rect 189 171 301 241 [[#12847]] | |||
rect 585 276 697 346 [[#12844]] | |||
rect 189 276 301 346 [[#12846]] | |||
desc none | |||
</imagemap> | |||
<imagemap> Bestand:LaboratoriumTest-v4.0Model(EN).png | center | |||
rect 177 35 332 85 [[#TestResultStatusCodelist]] | |||
rect 210 124 300 194 [[#12859]] | |||
rect 596 121 769 171 [[#InterpretationMethodCodelist]] | |||
rect 457 122 566 192 [[#12869]] | |||
rect 337 122 438 192 [[#12874]] | |||
rect 32 335 187 385 [[#TestMethodCodelist]] | |||
rect 32 251 187 301 [[#TestNameCodelist]] | |||
rect 312 528 482 578 [[#ResultFlagsCodelist]] | |||
rect 500 329 616 399 [[#12872]] | |||
rect 347 431 447 501 [[#12866]] | |||
rect 353 233 443 303 [[#12870]] | |||
rect 500 235 616 305 [[#12865]] | |||
rect 213 332 299 402 [[#12857]] | |||
rect 213 238 299 308 [[#12861]] | |||
rect 500 432 616 502 [[#12863]] | |||
rect 213 431 299 501 [[#12875]] | |||
desc none | |||
</imagemap> | |||
<imagemap> Bestand:Monster-v4.0Model(EN).png | center | |||
rect 259 603 413 653 [[#MicroorganismCodelist]] | |||
rect 97 600 238 650 [[#ContainerTypeCodelist]] | |||
rect 704 78 916 128 [[#SpecimenAnatomicalLocationCodelist]] | |||
rect 704 166 828 216 [[#LateralityCodelist]] | |||
rect 704 254 828 304 [[#MorphologyCodelist]] | |||
rect 281 69 391 139 [[#12882]] | |||
rect 551 155 661 225 [[#12884]] | |||
rect 551 330 661 400 [[#12880]] | |||
rect 146 287 256 357 [[#12879]] | |||
rect 146 505 256 575 [[#12885]] | |||
rect 146 396 256 466 [[#12894]] | |||
rect 281 505 391 575 [[#12876]] | |||
rect 416 69 526 139 [[#12886]] | |||
rect 551 69 661 139 [[#12892]] | |||
rect 554 244 664 314 [[#12889]] | |||
rect 426 603 576 653 [[#SpecimenMaterialCodelist]] | |||
rect 704 427 871 477 [[#CollectioMethodCodelist]] | |||
rect 341 286 431 356 [[#12891]] | |||
rect 551 417 661 487 [[#12887]] | |||
rect 417 504 527 574 [[#12896]] | |||
rect 146 178 256 248 [[#12898]] | |||
rect 551 504 661 574 [[#12881]] | |||
rect 146 69 256 139 [[#12883]] | |||
desc none | |||
</imagemap> | |||
<BR> | <BR> | ||
{| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | {| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | ||
| Regel 207: | Regel 263: | ||
|style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | |style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | ||
|-style="vertical-align:top; background-color: #E3E3E3; " | |-style="vertical-align:top; background-color: #E3E3E3; " | ||
|style = "text-align:center" |[[Bestand: block.png| 20px]] | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | ||
||NL-CM:13.1.1 | ||NL-CM:13.1.1 | ||
|colspan ="6" style ="padding-left: 0px"|<span Title="NL: LaboratoriumUitslag">[[Bestand: arrowdown.png | 10px]]LaboratoryTestResult</span> | |colspan ="6" style ="padding-left: 0px"|<span Id=12851 Title="NL: LaboratoriumUitslag">[[Bestand: arrowdown.png | 10px | link=]]LaboratoryTestResult</span> | ||
| | | | ||
|Root concept of the LaboratoryTestResult information model. This root concept contains all data elements of the LaboratoryTestResult information model. | |Root concept of the LaboratoryTestResult information model. This root concept contains all data elements of the LaboratoryTestResult information model. | ||
| Regel 215: | Regel 271: | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:13.1.2 | ||NL-CM:13.1.2 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Monster">[[Bestand: arrowdown.png | 10px]]Specimen</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12891 Title="NL: Monster">[[Bestand: arrowdown.png | 10px | link=]]Specimen</span> | ||
|0..1 | |0..1 | ||
|Container of the Specimen concept. This container contains all data elements of the Specimen concept. | |Container of the Specimen concept. This container contains all data elements of the Specimen concept. | ||
| Regel 228: | Regel 284: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: II.png| 16px]] | |style = "text-align:center" |[[Bestand: II.png| 16px | link=]] | ||
||NL-CM:13.1.15 | ||NL-CM:13.1.15 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Monsternummer">[[Bestand: arrowright.png | 10px]]SpecimenId</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12898 Title="NL: Monsternummer">[[Bestand: arrowright.png | 10px | link=]]SpecimenId</span> | ||
|0..* | |0..* | ||
|Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | |Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | ||
| Regel 238: | Regel 294: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: INT.png| 16px]] | |style = "text-align:center" |[[Bestand: INT.png| 16px | link=]] | ||
||NL-CM:13.1.20 | ||NL-CM:13.1.20 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Monstervolgnummer">[[Bestand: arrowright.png | 10px]]SpecimenNumberExtension</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12879 Title="NL: Monstervolgnummer">[[Bestand: arrowright.png | 10px | link=]]SpecimenNumberExtension</span> | ||
|0..1 | |0..1 | ||
|The specimen number extension is used when the collected material is distributed from the original tube or container across multiple tubes. In combination with the specimen Id the extension yield a unique identification of the tube or container | |The specimen number extension is used when the collected material is distributed from the original tube or container across multiple tubes. In combination with the specimen Id the extension yield a unique identification of the tube or container | ||
| Regel 248: | Regel 304: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.21 | ||NL-CM:13.1.21 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Containertype">[[Bestand: arrowright.png | 10px]]ContainerType</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12885 Title="NL: Containertype">[[Bestand: arrowright.png | 10px | link=]]ContainerType</span> | ||
|0..1 | |0..1 | ||
|Container type describes the envelope in which the material is collected or sent. Examples include blood tubes, transport container, possibly including culture medium. | |Container type describes the envelope in which the material is collected or sent. Examples include blood tubes, transport container, possibly including culture medium. | ||
| Regel 259: | Regel 315: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ContainerTypeCodelist|ContainerTypeCodelist]] | |[[Bestand: List2.png | link=#ContainerTypeCodelist]]||[[#ContainerTypeCodelist|ContainerTypeCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.16 | ||NL-CM:13.1.16 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Monstermateriaal">[[Bestand: arrowright.png | 10px]]SpecimenMaterial</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12896 Title="NL: Monstermateriaal">[[Bestand: arrowright.png | 10px | link=]]SpecimenMaterial</span> | ||
|1 | |1 | ||
|SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level. | |SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level. | ||
| Regel 281: | Regel 337: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#SpecimenMaterialCodelist|SpecimenMaterialCodelist]] | |[[Bestand: List2.png | link=#SpecimenMaterialCodelist]]||[[#SpecimenMaterialCodelist|SpecimenMaterialCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.22 | ||NL-CM:13.1.22 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Microorganisme">[[Bestand: arrowright.png | 10px]]Microorganism</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12876 Title="NL: Microorganisme">[[Bestand: arrowright.png | 10px | link=]]Microorganism</span> | ||
|0..1 | |0..1 | ||
|In particular in microbiological determinations the subject of the test is an isolate of certain microorganism rather then a material. This concept provides the ability to capture information about this microorganism. | |In particular in microbiological determinations the subject of the test is an isolate of certain microorganism rather then a material. This concept provides the ability to capture information about this microorganism. | ||
| Regel 295: | Regel 351: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#MicroorganismCodelist|MicroorganismCodelist]] | |[[Bestand: List2.png | link=#MicroorganismCodelist]]||[[#MicroorganismCodelist|MicroorganismCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: PQ.png| 16px]] | |style = "text-align:center" |[[Bestand: PQ.png| 16px | link=]] | ||
||NL-CM:13.1.23 | ||NL-CM:13.1.23 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Verzamelvolume">[[Bestand: arrowright.png | 10px]]CollectedVolume</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12894 Title="NL: Verzamelvolume">[[Bestand: arrowright.png | 10px | link=]]CollectedVolume</span> | ||
|0..1 | |0..1 | ||
|Total volume of the collected material. If it is necessary to determine the absolute amount of a particular substance in the collected material, the volume thereof must be given. | |Total volume of the collected material. If it is necessary to determine the absolute amount of a particular substance in the collected material, the volume thereof must be given. | ||
| Regel 308: | Regel 364: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:13.1.24 | ||NL-CM:13.1.24 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Verzamelperiode">[[Bestand: arrowright.png | 10px]]CollectionPeriod</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12886 Title="NL: Verzamelperiode">[[Bestand: arrowright.png | 10px | link=]]CollectionPeriod</span> | ||
|0..1 | |0..1 | ||
|If the material has not been collected at a single point in time but over a certain period, this period can be captured in this concept. An example is 24 hour urine. | |If the material has not been collected at a single point in time but over a certain period, this period can be captured in this concept. An example is 24 hour urine. | ||
| Regel 318: | Regel 374: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:13.1.17 | ||NL-CM:13.1.17 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: AfnameDatumTijd">[[Bestand: arrowright.png | 10px]]CollectionDateTime</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12883 Title="NL: AfnameDatumTijd">[[Bestand: arrowright.png | 10px | link=]]CollectionDateTime</span> | ||
|0..1 | |0..1 | ||
|Time at which the material was collected. | |Time at which the material was collected. | ||
| Regel 332: | Regel 388: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:13.1.25 | ||NL-CM:13.1.25 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: AannameDatumTijd">[[Bestand: arrowright.png | 10px]] | |colspan ="4" style ="padding-left: 0px"|<span Id=12882 Title="NL: AannameDatumTijd">[[Bestand: arrowright.png | 10px | link=]]ReceivedDateTime</span> | ||
|0..1 | |0..1 | ||
|Date and time that the material is handed over at the laboratory or specimen collection center. This is the issue with material that is collected by the patient himself. | |Date and time that the material is handed over at the laboratory or specimen collection center. This is the issue with material that is collected by the patient himself. | ||
| Regel 342: | Regel 398: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.18 | ||NL-CM:13.1.18 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Afnameprocedure">[[Bestand: arrowright.png | 10px]]CollectionMethod</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12887 Title="NL: Afnameprocedure">[[Bestand: arrowright.png | 10px | link=]]CollectionMethod</span> | ||
|0..1 | |0..1 | ||
|If relevant for the results, the method of obtaining the specimen can be entered as well. | |If relevant for the results, the method of obtaining the specimen can be entered as well. | ||
| Regel 357: | Regel 413: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#CollectioMethodCodelist|CollectioMethodCodelist]] | |[[Bestand: List2.png | link=#CollectioMethodCodelist]]||[[#CollectioMethodCodelist|CollectioMethodCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.26 | ||NL-CM:13.1.26 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: AnatomischeLocatie">[[Bestand: arrowright.png | 10px]]AnatomicalLocation</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12892 Title="NL: AnatomischeLocatie">[[Bestand: arrowright.png | 10px | link=]]AnatomicalLocation</span> | ||
|0..1 | |0..1 | ||
|Anatomic location where the material is collected, <i>e.g. </i>elbow | |Anatomic location where the material is collected, <i>e.g. </i>elbow | ||
| Regel 375: | Regel 431: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#SpecimenAnatomicalLocationCodelist|SpecimenAnatomicalLocationCodelist]] | |[[Bestand: List2.png | link=#SpecimenAnatomicalLocationCodelist]]||[[#SpecimenAnatomicalLocationCodelist|SpecimenAnatomicalLocationCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.27 | ||NL-CM:13.1.27 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Lateraliteit">[[Bestand: arrowright.png | 10px]]Laterality</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12884 Title="NL: Lateraliteit">[[Bestand: arrowright.png | 10px | link=]]Laterality</span> | ||
|0..1 | |0..1 | ||
|Laterality adds information about body side to the anatomic location, <i>e.g.</i> left | |Laterality adds information about body side to the anatomic location, <i>e.g.</i> left | ||
| Regel 393: | Regel 449: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#LateralityCodelist|LateralityCodelist]] | |[[Bestand: List2.png | link=#LateralityCodelist]]||[[#LateralityCodelist|LateralityCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.28 | ||NL-CM:13.1.28 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Morfologie">[[Bestand: arrowright.png | 10px]]Morphology</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12889 Title="NL: Morfologie">[[Bestand: arrowright.png | 10px | link=]]Morphology</span> | ||
|0..1 | |0..1 | ||
|Morphology describes morphological abnormalities of the anatomical location where the material is taken, for example wound, ulcer. | |Morphology describes morphological abnormalities of the anatomical location where the material is taken, for example wound, ulcer. | ||
| Regel 411: | Regel 467: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#MorphologyCodelist|MorphologyCodelist]] | |[[Bestand: List2.png | link=#MorphologyCodelist]]||[[#MorphologyCodelist|MorphologyCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:13.1.29 | ||NL-CM:13.1.29 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: BronMonster">[[Bestand: arrowright.png | 10px]]SpecimenSource</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12880 Title="NL: BronMonster">[[Bestand: arrowright.png | 10px | link=]]SpecimenSource</span> | ||
|0..1 | |0..1 | ||
|If the material is not collected directly from the patient but comes from a patient-related object, <i>e.g.</i> a cathetertip, this source of material can be recorded here. | |If the material is not collected directly from the patient but comes from a patient-related object, <i>e.g.</i> a cathetertip, this source of material can be recorded here. | ||
| Regel 428: | Regel 484: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:13.1.19 | ||NL-CM:13.1.19 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Comment</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12881 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Comment</span> | ||
|0..1 | |0..1 | ||
|Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. | |Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. | ||
| Regel 442: | Regel 498: | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:13.1.3 | ||NL-CM:13.1.3 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: LaboratoriumTest">[[Bestand: arrowdown.png | 10px]]LaboratoryTest</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12870 Title="NL: LaboratoriumTest">[[Bestand: arrowdown.png | 10px | link=]]LaboratoryTest</span> | ||
|0..* | |0..* | ||
|Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | |Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | ||
| Regel 451: | Regel 507: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.8 | ||NL-CM:13.1.8 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Test">[[Bestand: arrowright.png | 10px]]Test</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12861 Title="NL: Test">[[Bestand: arrowright.png | 10px | link=]]Test</span> | ||
|1 | |1 | ||
|The name and code of the executed test. | |The name and code of the executed test. | ||
| Regel 462: | Regel 518: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#TestNameCodelist|TestNameCodelist]] | |[[Bestand: List2.png | link=#TestNameCodelist]]||[[#TestNameCodelist|TestNameCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.9 | ||NL-CM:13.1.9 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Testmethode">[[Bestand: arrowright.png | 10px]]TestMethod</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12857 Title="NL: Testmethode">[[Bestand: arrowright.png | 10px | link=]]TestMethod</span> | ||
|0..1 | |0..1 | ||
|The test method used to obtain the result. | |The test method used to obtain the result. | ||
| Regel 480: | Regel 536: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#TestMethodCodelist|TestMethodCodelist]] | |[[Bestand: List2.png | link=#TestMethodCodelist]]||[[#TestMethodCodelist|TestMethodCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:13.1.13 | ||NL-CM:13.1.13 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: TestDatumTijd">[[Bestand: arrowright.png | 10px]]TestDateTime</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12875 Title="NL: TestDatumTijd">[[Bestand: arrowright.png | 10px | link=]]TestDateTime</span> | ||
|0..1 | |0..1 | ||
|The date and if possible the time at which the test was carried out. | |The date and if possible the time at which the test was carried out. | ||
| Regel 493: | Regel 549: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ANY.png| 16px]] | |style = "text-align:center" |[[Bestand: ANY.png| 16px | link=]] | ||
||NL-CM:13.1.10 | ||NL-CM:13.1.10 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Uitslag">[[Bestand: arrowright.png | 10px]]Result</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12865 Title="NL: Uitslag">[[Bestand: arrowright.png | 10px | link=]]Result</span> | ||
|1 | |1 | ||
|The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | |The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | ||
| Regel 503: | Regel 559: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.31 | ||NL-CM:13.1.31 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: TestUitslagStatus">[[Bestand: arrowright.png | 10px]]TestResultStatus</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12859 Title="NL: TestUitslagStatus">[[Bestand: arrowright.png | 10px | link=]]TestResultStatus</span> | ||
|0..1 | |0..1 | ||
|The status of the test result of this test. If the laboratory test is an panel/cluster, the overall status is given in the status of the panel/cluster. | |The status of the test result of this test. If the laboratory test is an panel/cluster, the overall status is given in the status of the panel/cluster. | ||
| Regel 514: | Regel 570: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#TestResultStatusCodelist|TestResultStatusCodelist]] | |[[Bestand: List2.png | link=#TestResultStatusCodelist]]||[[#TestResultStatusCodelist|TestResultStatusCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ANY.png| 16px]] | |style = "text-align:center" |[[Bestand: ANY.png| 16px | link=]] | ||
||NL-CM:13.1.11 | ||NL-CM:13.1.11 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ReferentieBovengrens">[[Bestand: arrowright.png | 10px]]ReferenceRangeUpperLimit</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12872 Title="NL: ReferentieBovengrens">[[Bestand: arrowright.png | 10px | link=]]ReferenceRangeUpperLimit</span> | ||
|0..1 | |0..1 | ||
|The upper reference limit for the patient of the value measured in the test. | |The upper reference limit for the patient of the value measured in the test. | ||
| Regel 527: | Regel 583: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ANY.png| 16px]] | |style = "text-align:center" |[[Bestand: ANY.png| 16px | link=]] | ||
||NL-CM:13.1.12 | ||NL-CM:13.1.12 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ReferentieOndergrens">[[Bestand: arrowright.png | 10px]]ReferenceRangeLowerLimit</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12863 Title="NL: ReferentieOndergrens">[[Bestand: arrowright.png | 10px | link=]]ReferenceRangeLowerLimit</span> | ||
|0..1 | |0..1 | ||
|The lower reference limit for the patient of the value measured with the test. | |The lower reference limit for the patient of the value measured with the test. | ||
| Regel 537: | Regel 593: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.30 | ||NL-CM:13.1.30 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: InterpretatieMethode">[[Bestand: arrowright.png | 10px]]InterpretationMethod</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12869 Title="NL: InterpretatieMethode">[[Bestand: arrowright.png | 10px | link=]]InterpretationMethod</span> | ||
|0..1 | |0..1 | ||
|The method used to determine interpretation flags. An example of this is EUCAST, for determining clinical breakpoints in microbiological susceptibility tests | |The method used to determine interpretation flags. An example of this is EUCAST, for determining clinical breakpoints in microbiological susceptibility tests | ||
| Regel 548: | Regel 604: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#InterpretationMethodCodelist|InterpretationMethodCodelist]] | |[[Bestand: List2.png | link=#InterpretationMethodCodelist]]||[[#InterpretationMethodCodelist|InterpretationMethodCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.14 | ||NL-CM:13.1.14 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: InterpretatieVlaggen">[[Bestand: arrowright.png | 10px]]ResultFlags</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12866 Title="NL: InterpretatieVlaggen">[[Bestand: arrowright.png | 10px | link=]]ResultFlags</span> | ||
|0..* | |0..* | ||
|Attention codes indicating whether the result is above or below certain reference values or interpreting the result otherwise.(Resistent) | |Attention codes indicating whether the result is above or below certain reference values or interpreting the result otherwise.(Resistent) | ||
| Regel 566: | Regel 622: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ResultFlagsCodelist|ResultFlagsCodelist]] | |[[Bestand: List2.png | link=#ResultFlagsCodelist]]||[[#ResultFlagsCodelist|ResultFlagsCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:13.1.32 | ||NL-CM:13.1.32 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: UitslagInterpretatie">[[Bestand: arrowright.png | 10px]]ResultInterpretation</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=12874 Title="NL: UitslagInterpretatie">[[Bestand: arrowright.png | 10px | link=]]ResultInterpretation</span> | ||
|0..1 | |0..1 | ||
|Comment of the laboratory specialist regarding the interpretation of the results | |Comment of the laboratory specialist regarding the interpretation of the results | ||
| Regel 583: | Regel 639: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.4 | ||NL-CM:13.1.4 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Onderzoek">[[Bestand: arrowright.png | 10px]]PanelOrBattery</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12847 Title="NL: Onderzoek">[[Bestand: arrowright.png | 10px | link=]]PanelOrBattery</span> | ||
|0..1 | |0..1 | ||
|For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. | |For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. | ||
| Regel 593: | Regel 649: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#PanelOrBatteryCodelist|PanelOrBatteryCodelist]] | |[[Bestand: List2.png | link=#PanelOrBatteryCodelist]]||[[#PanelOrBatteryCodelist|PanelOrBatteryCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.6 | ||NL-CM:13.1.6 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ResultaatStatus">[[Bestand: arrowright.png | 10px]]ResultStatus</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12846 Title="NL: ResultaatStatus">[[Bestand: arrowright.png | 10px | link=]]ResultStatus</span> | ||
|0..1 | |0..1 | ||
|The status of the laboratory test result .If the laboratory test is an panel/cluster, this status reflects the status of the whole panel/cluster. If the status item per subtest is used too, this status must be in accordance with these status values. | |The status of the laboratory test result .If the laboratory test is an panel/cluster, this status reflects the status of the whole panel/cluster. If the status item per subtest is used too, this status must be in accordance with these status values. | ||
| Regel 606: | Regel 662: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ResultStatusCodelist|ResultStatusCodelist]] | |[[Bestand: List2.png | link=#ResultStatusCodelist]]||[[#ResultStatusCodelist|ResultStatusCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:13.1.5 | ||NL-CM:13.1.5 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Comment</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12848 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Comment</span> | ||
|0..1 | |0..1 | ||
|Comments, such as a textual interpretation or advice accompanying the result, for example. | |Comments, such as a textual interpretation or advice accompanying the result, for example. | ||
| Regel 622: | Regel 678: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.7 | ||NL-CM:13.1.7 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ResultaatType">[[Bestand: arrowright.png | 10px]]ResultType</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12844 Title="NL: ResultaatType">[[Bestand: arrowright.png | 10px | link=]]ResultType</span> | ||
|1 | |1 | ||
|The type of result defines the laboratory specialty under which the test is categorized. | |The type of result defines the laboratory specialty under which the test is categorized. | ||
| Regel 632: | Regel 688: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ResultTypeCodelist|ResultTypeCodelist]] | |[[Bestand: List2.png | link=#ResultTypeCodelist]]||[[#ResultTypeCodelist|ResultTypeCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] | ||
||NL-CM:13.1.33 | ||NL-CM:13.1.33 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: GerelateerdeUitslag::LaboratoriumUitslag">[[Bestand: arrowright.png | 10px]]RelatedResult::LaboratoryTestResult</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=12849 Title="NL: GerelateerdeUitslag::LaboratoriumUitslag">[[Bestand: arrowright.png | 10px | link=]]RelatedResult::LaboratoryTestResult</span> | ||
|0..* | |0..* | ||
|Reference to related tests, <i>e.g.</i> paired tests or sequential tests like gram staining and microbiological cultures | |Reference to related tests, <i>e.g.</i> paired tests or sequential tests like gram staining and microbiological cultures | ||
| Regel 645: | Regel 701: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: block.png]]||[[LaboratoryTestResult-v4.0(2017EN) |LaboratoryTestResult]] | |[[Bestand: block.png | link=LaboratoryTestResult-v4.0(2017EN)]]||[[LaboratoryTestResult-v4.0(2017EN) |LaboratoryTestResult]] | ||
|} | |} | ||
|} | |} | ||
| Regel 656: | Regel 712: | ||
{|class="wikitable" width="1043px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1043px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
|colspan="10" style="background-color: #1F497D; width: 100%; color | |colspan="10" style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>LaboratoriumUitslag</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Type</b></font> | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Status</b></font> | ||
|colspan="2" style="background-color: #548DD4; width: 18%; color | |colspan="2" style="background-color: #548DD4; width: 18%; "|<font color=#FFFFFF><b>Monster</b></font> | ||
|colspan="6" style="background-color: #548DD4; width: 61%; color | |colspan="6" style="background-color: #548DD4; width: 61%; "|<font color=#FFFFFF><b>LaboratoriumTest</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| style="background-color: #D9D9D9; width: 10%; "| | | style="background-color: #D9D9D9; width: 10%; "| | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Monster</b><BR><b>materiaal</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Afname</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Uitslag</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Referentie</b><BR><b>Ondergrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Referentie</b><BR><b>Bovengrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Interpretatie</b><BR><b>Vlaggen</b> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Klinische chemie | | style="width: 11%; "|Klinische chemie | ||
| Regel 687: | Regel 743: | ||
{|class="wikitable" width="1043px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1043px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
|colspan="10" style="background-color: #1F497D; width: 100%; color | |colspan="10" style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>LaboratoriumUitslag</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Type</b></font> | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Status</b></font> | ||
|colspan="2" style="background-color: #548DD4; width: 18%; color | |colspan="2" style="background-color: #548DD4; width: 18%; "|<font color=#FFFFFF><b>Monster</b></font> | ||
|colspan="6" style="background-color: #548DD4; width: 61%; color | |colspan="6" style="background-color: #548DD4; width: 61%; "|<font color=#FFFFFF><b>LaboratoriumTest</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| style="background-color: #D9D9D9; width: 10%; "| | | style="background-color: #D9D9D9; width: 10%; "| | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Monster</b><BR><b>materiaal</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Afname</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Uitslag</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Referentie</b><BR><b>Ondergrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Referentie</b><BR><b>Bovengrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Interpretatie</b><BR><b>Vlaggen</b> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Klinische chemie | | style="width: 11%; "|Klinische chemie | ||
| Regel 718: | Regel 774: | ||
{|class="wikitable" width="1043px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1043px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
|colspan="10" style="background-color: #1F497D; width: 100%; color | |colspan="10" style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>LaboratoriumUitslag</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Type</b></font> | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Status</b></font> | ||
|colspan="2" style="background-color: #548DD4; width: 18%; color | |colspan="2" style="background-color: #548DD4; width: 18%; "|<font color=#FFFFFF><b>Monster</b></font> | ||
|colspan="6" style="background-color: #548DD4; width: 61%; color | |colspan="6" style="background-color: #548DD4; width: 61%; "|<font color=#FFFFFF><b>LaboratoriumTest</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| style="background-color: #D9D9D9; width: 10%; "| | | style="background-color: #D9D9D9; width: 10%; "| | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Monster</b><BR><b>materiaal</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Afname</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Uitslag</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Referentie</b><BR><b>Ondergrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Referentie</b><BR><b>Bovengrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Interpretatie</b><BR><b>Vlaggen</b> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Virologie | | style="width: 11%; "|Virologie | ||
| Regel 1.122: | Regel 1.178: | ||
==This information model in other releases== | |||
<ul> | |||
<li>[[LaboratoryTestResultForTransfer-v1.2.2(2015EN) | Release 2015, (Version 1.2.2)]]</li> | |||
<li>[[LaboratoryTestResultForTransfer-v3.0(2016EN) | Release 2016, (Version 3.0)]]</li> | |||
</ul> | |||
==More on this information model== | ==More on this information model== | ||
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | |||
This information model is also available as [[Media:nl.zorg.LaboratoryTestResult-v4.0( | Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications: | ||
<ul> | |||
<li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment [http://decor.nictiz.nl/art-decor/decor-scenarios--zib2017bbr-?id=2.16.840.1.113883.2.4.3.11.60.7.4.2.13.1&effectiveDate=2017-09-04T00:00:00&language=en-US&scenariotree=false [[File:artdecor.jpg|16px|link=]]]</li> | |||
<li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR [https://simplifier.net/NictizSTU3/~resources?text=zib&category=Profile [[File:fhir.png|link=]]]</li> | |||
</ul> | |||
This information model is also available as [[Media:nl.zorg.LaboratoryTestResult-v4.0(2017EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.LaboratoryTestResult-v4.0(2017EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | |||
==About this information== | ==About this information== | ||
The information in this wikipage is based on Prerelease 2017 #1 <BR> | The information in this wikipage is based on Prerelease 2017 #1 <BR> | ||
Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | ||
This page is generated on | This page is generated on 30/11/2017 13:37:14 <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | ||
Huidige versie van 20 feb 2018 om 10:05
General information
Name: nl.zorg.LaboratoryTestResult ![]()
Version: 4.0
HCIM Status:Final
Release: 2017
Release status: Prepublished
Release date: 04-09-2017
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 7-6-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.13.1 |
| DCM::KeywordList | laboratorium uitslag, lab, laboratorium bepaling |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.LaboratoriumUitslag |
| DCM::PublicationDate | 04-09-2017 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 04-09-2017 |
| DCM::Superseeds | nl.zorg.OverdrachtLaboratoriumUitslag-v3.0 |
| DCM::Version | 4.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-238 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept TestNaam opsplitsen. |
| ZIB-239 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet SNOMED - eLab valueset van concept Testmethode opsplitsen. |
| ZIB-240 | In de klinische bouwsteen OverdrachtLabUitslag kwam de tagged value DCM::ValueSet van concept LaboratoriumTest niet overeen met de naam van de gekoppelde waardenlijst ResultNormalcyStatus Valueset (HL7). |
| ZIB-241 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept Onderzoek opsplitsen. |
| ZIB-242 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatStatus niet overeen met de tagged value van het concept. |
| ZIB-243 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatType niet overeen met de tagged value van het concept. |
| ZIB-244 | Tagged values van concept Onderzoek van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-245 | Tagged values van concept Testmethode van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-246 | Tagged values van concept TestNaam van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
| ZIB-361 | Naamgeving concept Opmerking aangepast |
| ZIB-367 | Opschonen ResultaatVlaggenCodelijst |
| ZIB-370 | ResultaatStatusCodelijst en TekstUitslagCodelijst codes aanpassen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 1.2.1 (22-05-2015)
| ZIB-392 | De ResultaatTypeCodelijst heeft geen "OID: " aanduiding in de onderliggende codelijst. |
Publicatieversie 1.2.2 (16-07-2015)
| ZIB-420 | Vervallen SNOMED CT code in ResultaatTypeCodelijst |
Publicatieversie 3.0 (01-05-2016)
| ZIB-423 | Verkeerd bron-codestelsel gekoppeld aan de 'TestStatusCodelijst'. |
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
Publicatieversie 4.0 (04-09-2017)
| ZIB-479 | Monster herkomst ontbreekt |
| ZIB-549 | De Engelse naam van de bouwsteen en het rootconcept zijn niet correct |
| ZIB-564 | Aanpassing/harmonisatie Engelse conceptnamen |
| ZIB-576 | Aanpassen bouwstenen die nog de prefix overdracht hebben, zodat de prefix kan vervallen. |
| ZIB-481 | Ambiguïteit in interpretatie van ResultaatStatus |
| ZIB-577 | Toevoegen SNOMED CT concept in ResultaatTypeCodelijst |
Concept
A laboratory result describes the result of a laboratory analysis.
These are specimen-oriented tests as performed in laboratories such as Clinical Chemistry, Serology, Microbiology, etc.
In addition to the results of tests with a singular result, this concept can also contain the results of more complex tests with multiple results or a ‘panel’.
Purpose
Laboratory tests are done for the purpose of diagnosing and preventing disease and follow-up on the effects of treatment.
Evidence Base
There are two information models for recording laboratory test results: TextResult and LaboratoryTestResult.
In the case of laboratory test results, it is difficult to clearly indicate exactly when to use this information model and when to use the TextResult information model.
In general, laboratory tests resulting in a value (7.1 mmol/L), ordinal number (++ from series to ++++) or a quantitative result (Low) are recorded using this information model. The TextResult information model is better suited for textual results that are more descriptive in nature and which are longer than just a few words. Both types of tests occur in almost all laboratories.
The applicability of the aforementioned information models is not determined by the kind of lab but by the kind of result.
Information Model
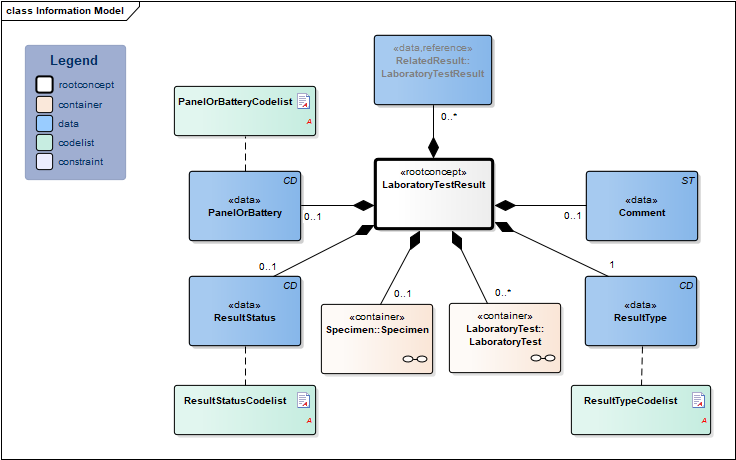
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | ||||||||
| NL-CM:13.1.1 | Root concept of the LaboratoryTestResult information model. This root concept contains all data elements of the LaboratoryTestResult information model. | |||||||||||||
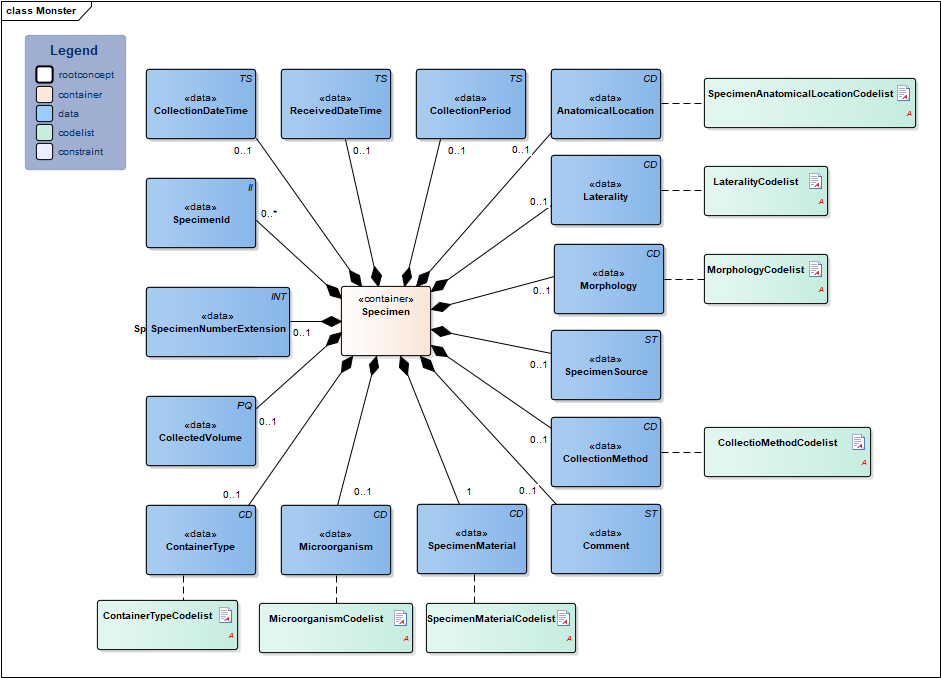
| NL-CM:13.1.2 | 0..1 | Container of the Specimen concept. This container contains all data elements of the Specimen concept. |
|
|||||||||||
| NL-CM:13.1.15 | 0..* | Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | ||||||||||||
| NL-CM:13.1.20 | 0..1 | The specimen number extension is used when the collected material is distributed from the original tube or container across multiple tubes. In combination with the specimen Id the extension yield a unique identification of the tube or container | ||||||||||||
| NL-CM:13.1.21 | 0..1 | Container type describes the envelope in which the material is collected or sent. Examples include blood tubes, transport container, possibly including culture medium. |
| |||||||||||
| NL-CM:13.1.16 | 1 | SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level.
This is in line with the agreements made in the IHE/Nictiz program e-Lab. If the test is carried out on derived material (such as plasma), this element will still contain the material drawn (in this case, blood). In this case, the LOINC code will generally refer to plasma. |
|
| ||||||||||
| NL-CM:13.1.22 | 0..1 | In particular in microbiological determinations the subject of the test is an isolate of certain microorganism rather then a material. This concept provides the ability to capture information about this microorganism. |
| |||||||||||
| NL-CM:13.1.23 | 0..1 | Total volume of the collected material. If it is necessary to determine the absolute amount of a particular substance in the collected material, the volume thereof must be given. | ||||||||||||
| NL-CM:13.1.24 | 0..1 | If the material has not been collected at a single point in time but over a certain period, this period can be captured in this concept. An example is 24 hour urine. | ||||||||||||
| NL-CM:13.1.17 | 0..1 | Time at which the material was collected. |
|
|||||||||||
| NL-CM:13.1.25 | 0..1 | Date and time that the material is handed over at the laboratory or specimen collection center. This is the issue with material that is collected by the patient himself. | ||||||||||||
| NL-CM:13.1.18 | 0..1 | If relevant for the results, the method of obtaining the specimen can be entered as well. |
|
| ||||||||||
| NL-CM:13.1.26 | 0..1 | Anatomic location where the material is collected, e.g. elbow |
|
| ||||||||||
| NL-CM:13.1.27 | 0..1 | Laterality adds information about body side to the anatomic location, e.g. left |
|
| ||||||||||
| NL-CM:13.1.28 | 0..1 | Morphology describes morphological abnormalities of the anatomical location where the material is taken, for example wound, ulcer. |
|
| ||||||||||
| NL-CM:13.1.29 | 0..1 | If the material is not collected directly from the patient but comes from a patient-related object, e.g. a cathetertip, this source of material can be recorded here. |
|
|||||||||||
| NL-CM:13.1.19 | 0..1 | Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. |
|
|||||||||||
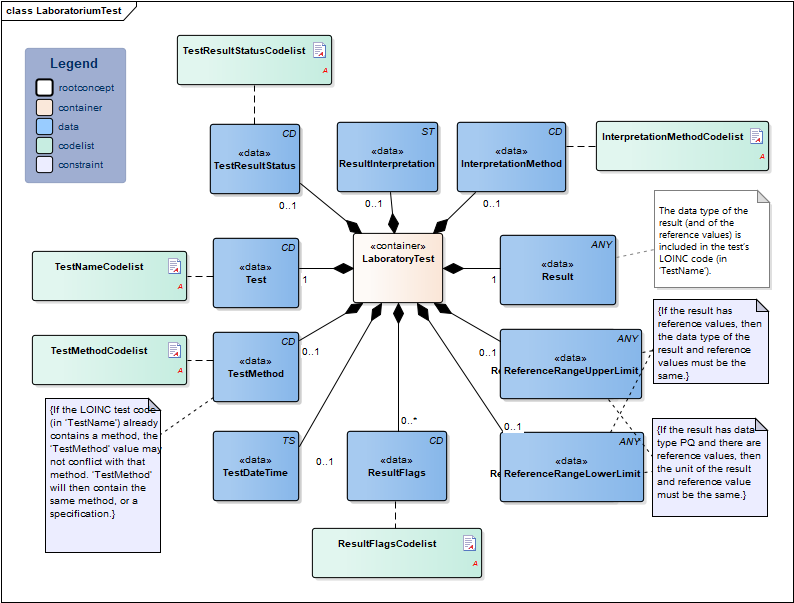
| NL-CM:13.1.3 | 0..* | Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | ||||||||||||
| NL-CM:13.1.8 | 1 | The name and code of the executed test. |
| |||||||||||
| NL-CM:13.1.9 | 0..1 | The test method used to obtain the result. |
|
| ||||||||||
| NL-CM:13.1.13 | 0..1 | The date and if possible the time at which the test was carried out. | ||||||||||||
| NL-CM:13.1.10 | 1 | The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | ||||||||||||
| NL-CM:13.1.31 | 0..1 | The status of the test result of this test. If the laboratory test is an panel/cluster, the overall status is given in the status of the panel/cluster. |
| |||||||||||
| NL-CM:13.1.11 | 0..1 | The upper reference limit for the patient of the value measured in the test. | ||||||||||||
| NL-CM:13.1.12 | 0..1 | The lower reference limit for the patient of the value measured with the test. | ||||||||||||
| NL-CM:13.1.30 | 0..1 | The method used to determine interpretation flags. An example of this is EUCAST, for determining clinical breakpoints in microbiological susceptibility tests |
| |||||||||||
| NL-CM:13.1.14 | 0..* | Attention codes indicating whether the result is above or below certain reference values or interpreting the result otherwise.(Resistent) |
|
| ||||||||||
| NL-CM:13.1.32 | 0..1 | Comment of the laboratory specialist regarding the interpretation of the results |
|
|||||||||||
| NL-CM:13.1.4 | 0..1 | For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. |
| |||||||||||
| NL-CM:13.1.6 | 0..1 | The status of the laboratory test result .If the laboratory test is an panel/cluster, this status reflects the status of the whole panel/cluster. If the status item per subtest is used too, this status must be in accordance with these status values. |
| |||||||||||
| NL-CM:13.1.5 | 0..1 | Comments, such as a textual interpretation or advice accompanying the result, for example. |
|
|||||||||||
| NL-CM:13.1.7 | 1 | The type of result defines the laboratory specialty under which the test is categorized. |
| |||||||||||
| NL-CM:13.1.33 | 0..* | Reference to related tests, e.g. paired tests or sequential tests like gram staining and microbiological cultures |
| |||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
Test | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 12-06-2012 09:00 | Natrium | 12-06-2012 13:15 | 138 mmol/l | 136 mmol/l | 146 mmol/l | |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
Test | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 23-05-2012 08:08 | Chloride | 23-05-2012 12:00 | 109 mmol/l | 99 mmol/l | 108 mmol/l | Boven referentie- waarde |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
Test | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Interpretatie Vlaggen | ||
| Virologie | Definitief | Bloed | 16-01-2012 08:00 | Hepatitis A IgM | 16-01-2012 10:12 | Negatief | |||
References
1. Nederlandse Vereniging voor Medische Microbiologie (2010) ELab en EvT. [Online] Beschikbaar op: http://www.nvmm.nl/ict/vereniging/werkgroepen_commissies/elab-en-evt [Geraadpleegd: 23 juli 2014].
Valuesets
CollectioMethodCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <17636008 | specimen collection | | SNOMED CT | 2.16.840.1.113883.6.96 |
ContainerTypeCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.9 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 434711009 |Specimen container (physical object)| | SNOMED CT | 2.16.840.1.113883.6.96 |
InterpretationMethodCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.14 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| EUCAST | tbd | SNOMED CT | 2.16.840.1.113883.6.96 | EUCAST |
LateralityCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.12 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Left | 7771000 | SNOMED CT | 2.16.840.1.113883.6.96 | Links |
| Right | 24028007 | SNOMED CT | 2.16.840.1.113883.6.96 | Rechts |
| Right and left | 51440002 | SNOMED CT | 2.16.840.1.113883.6.96 | Rechts en links |
MicroorganismCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.10 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: ^2581000146104 |simpele referentieset voor micro-organismen (foundation metadata concept)| | SNOMED CT | 2.16.840.1.113883.6.96 |
MorphologyCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.13 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 49755003 |Morphologically abnormal structure| | SNOMED CT | 2.16.840.1.113883.6.96 |
PanelOrBatteryCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
ResultFlagsCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Above reference range | 281302008 | SNOMED CT | 2.16.840.1.113883.6.96 | Boven referentiewaarde |
| Below reference range | 281300000 | SNOMED CT | 2.16.840.1.113883.6.96 | Onder referentiewaarde |
| Intermediate | 11896004 | SNOMED CT | 2.16.840.1.113883.6.96 | Intermediair |
| Resistant | 30714006 | SNOMED CT | 2.16.840.1.113883.6.96 | Resistent |
| Susceptible | 131196009 | SNOMED CT | 2.16.840.1.113883.6.96 | Sensitief |
ResultStatusCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
ResultTypeCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Hematology | 252275004 | SNOMED CT | 2.16.840.1.113883.6.96 | Hematologie |
| Chemistry | 275711006 | SNOMED CT | 2.16.840.1.113883.6.96 | Klinische chemie |
| Serology | 68793005 | SNOMED CT | 2.16.840.1.113883.6.96 | Serologie/ immunologie |
| Virology | 395124008 | SNOMED CT | 2.16.840.1.113883.6.96 | Virologie |
| Toxicology | 314076009 | SNOMED CT | 2.16.840.1.113883.6.96 | Toxicologie |
| Microbiology | 19851009 | SNOMED CT | 2.16.840.1.113883.6.96 | Microbiologie |
| Molecular genetics | 405825005 | SNOMED CT | 2.16.840.1.113883.6.96 | Moleculaire genetica |
SpecimenAnatomicalLocationCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.11 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 442083009 |Anatomical or acquired body structure| | SNOMED CT | 2.16.840.1.113883.6.96 |
SpecimenMaterialCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 105590001 |substantie| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestMethodCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: < 272394005 |Technique (qualifier value)| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestNameCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
TestResultStatusCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.15 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
This information model in other releases
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2017 #1
Conditions for use are located on the mainpage ![]()
This page is generated on 30/11/2017 13:37:14