LaboratoryTestResultForTransfer-v3.0(2016EN): verschil tussen versies
Nieuwe pagina aangemaakt met '==General information== Name: '''nl.zorg.LaboratoryTestResultForTransfer''' link=OverdrachtLaboratoriumUitslag-v3.0(2016NL)<BR> Version: '''3.0...' |
Geen bewerkingssamenvatting |
||
| (3 tussenliggende versies door dezelfde gebruiker niet weergegeven) | |||
| Regel 1: | Regel 1: | ||
==General information== | <!-- Hieronder wordt een transclude page aangeroepen --> | ||
Name: '''nl.zorg.LaboratoryTestResultForTransfer''' [[Bestand:NL.png|link=OverdrachtLaboratoriumUitslag-v3.0(2016NL)]]<BR> | {{Versions-2.16.840.1.113883.2.4.3.11.60.40.3.13.1(EN)|1|LaboratoryTestResultForTransfer-v3.0(2016EN)}} | ||
Version: '''3.0''' <br> | <!-- Tot hier de transclude page --> | ||
HCIM Status:Final<br> | ==General information<!--hdGeneralInformation-->== | ||
Name<!--hdName-->: '''nl.zorg.LaboratoryTestResultForTransfer''' [[Bestand:NL.png|link=OverdrachtLaboratoriumUitslag-v3.0(2016NL)]]<BR> | |||
Release status: Published<br> | Version<!--hdVersion-->: '''3.0''' <br> | ||
HCIM Status<!--hdStatus-->:Final<br> | |||
Release<!--hdPublication-->: '''2016''' <br> | |||
Release status<!--hdPublicationStatus-->: Published<br> | |||
Release date<!--hdPublicationDate-->: 1-5-2016 | |||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2016(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2016(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | ||
==Metadata== | ==Metadata== | ||
| Regel 61: | Regel 64: | ||
==Revision History== | ==Revision History== | ||
<div class="mw-collapsible mw-collapsed"> | <div class="mw-collapsible mw-collapsed"> | ||
''Only available in Dutch'' | ''Only available in Dutch<!--noTranslation-->'' | ||
<u>Publicatieversie 1.0</u> (15-02-2013) | <u>Publicatieversie 1.0</u> (15-02-2013) | ||
| Regel 177: | Regel 180: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
<imagemap> Bestand:LaboratoryTestResultForTransfer-v3.0Model(2016EN).png | center | |||
[[Bestand: LaboratoriumTest-v3.0Model( | rect 225 378 367 428 [[#ResultStatusCodelist]] | ||
[[Bestand: Monster-v3.0Model( | rect 68 173 211 223 [[#TestCodelist]] | ||
rect 637 164 749 234 [[#10897]] | |||
rect 371 297 483 367 [[#10919]] | |||
rect 624 378 764 428 [[#ResultTypeCodelist]] | |||
rect 426 152 545 222 [[#10899]] | |||
rect 499 297 611 367 [[#10911]] | |||
rect 240 164 352 234 [[#10896]] | |||
rect 636 269 748 339 [[#10892]] | |||
rect 240 269 352 339 [[#10895]] | |||
desc none | |||
</imagemap> | |||
<imagemap> Bestand:LaboratoriumTest-v3.0Model(2016EN).png | center | |||
rect 35 104 190 154 [[#TestNameCodelist]] | |||
rect 35 188 190 238 [[#TestMethodCodelist]] | |||
rect 325 381 477 431 [[#ResultFlagsCodelist]] | |||
rect 503 182 619 252 [[#10913]] | |||
rect 351 284 451 354 [[#10910]] | |||
rect 356 86 446 156 [[#10911]] | |||
rect 503 88 619 158 [[#10909]] | |||
rect 216 185 302 255 [[#10903]] | |||
rect 216 91 302 161 [[#10905]] | |||
rect 503 285 619 355 [[#10907]] | |||
rect 216 284 302 354 [[#10915]] | |||
desc none | |||
</imagemap> | |||
<imagemap> Bestand:Monster-v3.0Model(2016EN).png | center | |||
rect 408 414 561 464 [[#DrawingProcedureCodelist]] | |||
rect 169 414 320 464 [[#SpecimenMaterialCodelist]] | |||
rect 311 115 401 185 [[#10919]] | |||
rect 435 305 525 375 [[#10918]] | |||
rect 195 305 285 375 [[#10920]] | |||
rect 145 179 235 249 [[#10921]] | |||
rect 480 179 570 249 [[#10916]] | |||
rect 314 305 404 375 [[#10917]] | |||
desc none | |||
</imagemap> | |||
<BR> | <BR> | ||
{| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | {| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; font-variant: small-caps; " | |-style="background-color: #1F497D; color: white; font-weight: bold; font-variant: small-caps; " | ||
|style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | |style="width:30px;"|Type<!--imType-->||style="width:100px;"|Id<!--imId-->||colspan="6 " style="width:140px;"|Concept<!--imConcept-->||Card.<!--imCard-->||style="width: 600px;"|Definition<!--imDefinition-->||style="width:200px;"|DefinitionCode<!--imDefinitionCode-->||style="width:200px;"|Reference<!--imReference--> | ||
|-style="vertical-align:top; background-color: #E3E3E3; " | |-style="vertical-align:top; background-color: #E3E3E3; " | ||
|style = "text-align:center" |[[Bestand: block.png| 20px]] | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | ||
||NL-CM:13.1.1 | ||NL-CM:13.1.1 | ||
|colspan ="6" style ="padding-left: 0px"|<span Title="NL: LaboratoriumUitslag">[[Bestand: arrowdown.png | 10px]]LaboratoryTestResult</span> | |colspan ="6" style ="padding-left: 0px"|<span Id=10899 Title="NL: LaboratoriumUitslag">[[Bestand: arrowdown.png | 10px | link=]]LaboratoryTestResult</span> | ||
| | | | ||
|Root concept of the LaboratoryTestResultTransfer information model. This root concept contains all data elements of the Laboratory TestResultTransfer information model. | |Root concept of the LaboratoryTestResultTransfer information model. This root concept contains all data elements of the Laboratory TestResultTransfer information model. | ||
| Regel 193: | Regel 231: | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:13.1.2 | ||NL-CM:13.1.2 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Monster">[[Bestand: arrowdown.png | 10px]]Specimen</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=10919 Title="NL: Monster">[[Bestand: arrowdown.png | 10px | link=]]Specimen</span> | ||
|1 | |1 | ||
|Container of the Specimen concept. This container contains all data elements of the Specimen concept. | |Container of the Specimen concept. This container contains all data elements of the Specimen concept. | ||
| Regel 202: | Regel 240: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: II.png| 16px]] | |style = "text-align:center" |[[Bestand: II.png| 16px | link=]] | ||
||NL-CM:13.1.15 | ||NL-CM:13.1.15 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Monsternummer">[[Bestand: arrowright.png | 10px]]SpecimenNumber</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10921 Title="NL: Monsternummer">[[Bestand: arrowright.png | 10px | link=]]SpecimenNumber</span> | ||
|0..* | |0..* | ||
|Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | |Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | ||
| Regel 212: | Regel 250: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.16 | ||NL-CM:13.1.16 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Monstermateriaal">[[Bestand: arrowright.png | 10px]]SpecimenMaterial</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10920 Title="NL: Monstermateriaal">[[Bestand: arrowright.png | 10px | link=]]SpecimenMaterial</span> | ||
|1 | |1 | ||
|SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level. | |SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level. | ||
| Regel 227: | Regel 265: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#SpecimenMaterialCodelist|SpecimenMaterialCodelist]] | |[[Bestand: List2.png | link=#SpecimenMaterialCodelist]]||[[#SpecimenMaterialCodelist|SpecimenMaterialCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:13.1.17 | ||NL-CM:13.1.17 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: AfnameDatumTijd">[[Bestand: arrowright.png | 10px]]DrawingDateTime</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10917 Title="NL: AfnameDatumTijd">[[Bestand: arrowright.png | 10px | link=]]DrawingDateTime</span> | ||
|1 | |1 | ||
|Time at which the material was drawn. | |Time at which the material was drawn. | ||
| Regel 240: | Regel 278: | ||
{| | {| | ||
|- | |- | ||
|<span | |<span title = "Codesystem: SNOMED CT>[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=399445004 399445004] specimen collection date</span> | ||
|} | |} | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.18 | ||NL-CM:13.1.18 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Afnameprocedure">[[Bestand: arrowright.png | 10px]]DrawingProcedure</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10918 Title="NL: Afnameprocedure">[[Bestand: arrowright.png | 10px | link=]]DrawingProcedure</span> | ||
|0..1 | |0..1 | ||
|If relevant for the results, the method of obtaining the specimen can be entered as well. | |If relevant for the results, the method of obtaining the specimen can be entered as well. | ||
| Regel 255: | Regel 293: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#DrawingProcedureCodelist|DrawingProcedureCodelist]] | |[[Bestand: List2.png | link=#DrawingProcedureCodelist]]||[[#DrawingProcedureCodelist|DrawingProcedureCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:13.1.19 | ||NL-CM:13.1.19 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Explanation</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10916 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Explanation</span> | ||
|0..1 | |0..1 | ||
|Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. | |Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. | ||
| Regel 268: | Regel 306: | ||
{| | {| | ||
|- | |- | ||
|<span | |<span title = "Codesystem: LOINC>[https://terminologie.nictiz.nl/art-decor/loinc?conceptId=48767-8 48767-8] Annotation comment</span> | ||
|} | |} | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:13.1.3 | ||NL-CM:13.1.3 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: LaboratoriumTest">[[Bestand: arrowdown.png | 10px]]LaboratoryTest</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=10911 Title="NL: LaboratoriumTest">[[Bestand: arrowdown.png | 10px | link=]]LaboratoryTest</span> | ||
|0..* | |0..* | ||
|Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | |Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | ||
| Regel 281: | Regel 319: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.8 | ||NL-CM:13.1.8 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: TestNaam">[[Bestand: arrowright.png | 10px]]TestName</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10905 Title="NL: TestNaam">[[Bestand: arrowright.png | 10px | link=]]TestName</span> | ||
|1 | |1 | ||
|The TestName is the name of the executed test. | |The TestName is the name of the executed test. | ||
| Regel 292: | Regel 330: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#TestNameCodelist|TestNameCodelist]] | |[[Bestand: List2.png | link=#TestNameCodelist]]||[[#TestNameCodelist|TestNameCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.9 | ||NL-CM:13.1.9 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Testmethode">[[Bestand: arrowright.png | 10px]]TestMethod</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10903 Title="NL: Testmethode">[[Bestand: arrowright.png | 10px | link=]]TestMethod</span> | ||
|0..1 | |0..1 | ||
|The test method used to obtain the result. | |The test method used to obtain the result. | ||
| Regel 306: | Regel 344: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#TestMethodCodelist|TestMethodCodelist]] | |[[Bestand: List2.png | link=#TestMethodCodelist]]||[[#TestMethodCodelist|TestMethodCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:13.1.13 | ||NL-CM:13.1.13 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: TestDatumTijd">[[Bestand: arrowright.png | 10px]]TestDateTime</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10915 Title="NL: TestDatumTijd">[[Bestand: arrowright.png | 10px | link=]]TestDateTime</span> | ||
|0..1 | |0..1 | ||
|The date and if possible the time at which the test was carried out. | |The date and if possible the time at which the test was carried out. | ||
| Regel 319: | Regel 357: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ANY.png| 16px]] | |style = "text-align:center" |[[Bestand: ANY.png| 16px | link=]] | ||
||NL-CM:13.1.10 | ||NL-CM:13.1.10 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Uitslag">[[Bestand: arrowright.png | 10px]]Result</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10909 Title="NL: Uitslag">[[Bestand: arrowright.png | 10px | link=]]Result</span> | ||
|1 | |1 | ||
|The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | |The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | ||
| Regel 329: | Regel 367: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ANY.png| 16px]] | |style = "text-align:center" |[[Bestand: ANY.png| 16px | link=]] | ||
||NL-CM:13.1.11 | ||NL-CM:13.1.11 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ReferentieBovengrens">[[Bestand: arrowright.png | 10px]]UpperReferenceLimit</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10913 Title="NL: ReferentieBovengrens">[[Bestand: arrowright.png | 10px | link=]]UpperReferenceLimit</span> | ||
|0..1 | |0..1 | ||
|The upper reference limit for the patient of the value measured in the test. | |The upper reference limit for the patient of the value measured in the test. | ||
| Regel 339: | Regel 377: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ANY.png| 16px]] | |style = "text-align:center" |[[Bestand: ANY.png| 16px | link=]] | ||
||NL-CM:13.1.12 | ||NL-CM:13.1.12 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ReferentieOndergrens">[[Bestand: arrowright.png | 10px]]LowerReferenceLimit</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10907 Title="NL: ReferentieOndergrens">[[Bestand: arrowright.png | 10px | link=]]LowerReferenceLimit</span> | ||
|0..1 | |0..1 | ||
|The lower reference limit for the patient of the value measured with the test. | |The lower reference limit for the patient of the value measured with the test. | ||
| Regel 349: | Regel 387: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.14 | ||NL-CM:13.1.14 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ResultaatVlaggen">[[Bestand: arrowright.png | 10px]]ResultFlags</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=10910 Title="NL: ResultaatVlaggen">[[Bestand: arrowright.png | 10px | link=]]ResultFlags</span> | ||
|0..* | |0..* | ||
|Attention codes indicating whether the result is above or below certain reference values. | |Attention codes indicating whether the result is above or below certain reference values. | ||
| Regel 360: | Regel 398: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ResultFlagsCodelist|ResultFlagsCodelist]] | |[[Bestand: List2.png | link=#ResultFlagsCodelist]]||[[#ResultFlagsCodelist|ResultFlagsCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.4 | ||NL-CM:13.1.4 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Onderzoek">[[Bestand: arrowright.png | 10px]]Test</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=10896 Title="NL: Onderzoek">[[Bestand: arrowright.png | 10px | link=]]Test</span> | ||
|0..1 | |0..1 | ||
|For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. | |For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. | ||
| Regel 373: | Regel 411: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#TestCodelist|TestCodelist]] | |[[Bestand: List2.png | link=#TestCodelist]]||[[#TestCodelist|TestCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.6 | ||NL-CM:13.1.6 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ResultaatStatus">[[Bestand: arrowright.png | 10px]]ResultStatus</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=10895 Title="NL: ResultaatStatus">[[Bestand: arrowright.png | 10px | link=]]ResultStatus</span> | ||
|0..1 | |0..1 | ||
|The status of the laboratory test result. | |The status of the laboratory test result. | ||
| Regel 386: | Regel 424: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ResultStatusCodelist|ResultStatusCodelist]] | |[[Bestand: List2.png | link=#ResultStatusCodelist]]||[[#ResultStatusCodelist|ResultStatusCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:13.1.5 | ||NL-CM:13.1.5 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Explanation</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=10897 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Explanation</span> | ||
|0..1 | |0..1 | ||
|Comments, such as a textual interpretation or advice accompanying the result, for example. | |Comments, such as a textual interpretation or advice accompanying the result, for example. | ||
| Regel 398: | Regel 436: | ||
{| | {| | ||
|- | |- | ||
|<span | |<span title = "Codesystem: LOINC>[https://terminologie.nictiz.nl/art-decor/loinc?conceptId=48767-8 48767-8] Annotation comment</span> | ||
|} | |} | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:13.1.7 | ||NL-CM:13.1.7 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ResultaatType">[[Bestand: arrowright.png | 10px]]ResultType</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=10892 Title="NL: ResultaatType">[[Bestand: arrowright.png | 10px | link=]]ResultType</span> | ||
|1 | |1 | ||
|The type of result defines the laboratory specialty under which the test is categorized. | |The type of result defines the laboratory specialty under which the test is categorized. | ||
| Regel 412: | Regel 450: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ResultTypeCodelist|ResultTypeCodelist]] | |[[Bestand: List2.png | link=#ResultTypeCodelist]]||[[#ResultTypeCodelist|ResultTypeCodelist]] | ||
|} | |} | ||
|} | |} | ||
Columns Concept and DefinitionCode: hover over the values for more information<BR> | Columns Concept and DefinitionCode: hover over the values for more information<!--imHover--><BR> | ||
For explanation of the symbols, please see the legend page [[Bestand:list2.png|link=Legend]] | For explanation of the symbols, please see the legend page<!--imLegend--> [[Bestand:list2.png|link=Legend<!--wikiLegend-->]] | ||
==Example Instances== | ==Example Instances== | ||
''Only available in Dutch'' | ''Only available in Dutch<!--noTranslation-->'' | ||
{|class="wikitable" width="1021px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1021px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
|colspan="10" style="background-color: #1F497D; width: 100%; color | |colspan="10" style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>LaboratoriumUitslag</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Type</b></font> | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Status</b></font> | ||
|colspan="2" style="background-color: #548DD4; width: 19%; color | |colspan="2" style="background-color: #548DD4; width: 19%; "|<font color=#FFFFFF><b>Monster</b></font> | ||
|colspan="6" style="background-color: #548DD4; width: 60%; color | |colspan="6" style="background-color: #548DD4; width: 60%; "|<font color=#FFFFFF><b>LaboratoriumTest</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| style="background-color: #D9D9D9; width: 10%; "| | | style="background-color: #D9D9D9; width: 10%; "| | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Monster</b><BR><b>materiaal</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Afname</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>TestNaam</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Uitslag</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Referentie</b><BR><b>Ondergrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Referentie</b><BR><b>Bovengrens</b> | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Resultaat</b><BR><b>Vlaggen</b> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Klinische chemie | | style="width: 11%; "|Klinische chemie | ||
| Regel 454: | Regel 492: | ||
{|class="wikitable" width="1021px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1021px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
|colspan="10" style="background-color: #1F497D; width: 100%; color | |colspan="10" style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>LaboratoriumUitslag</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Type</b></font> | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Status</b></font> | ||
|colspan="2" style="background-color: #548DD4; width: 19%; color | |colspan="2" style="background-color: #548DD4; width: 19%; "|<font color=#FFFFFF><b>Monster</b></font> | ||
|colspan="6" style="background-color: #548DD4; width: 60%; color | |colspan="6" style="background-color: #548DD4; width: 60%; "|<font color=#FFFFFF><b>LaboratoriumTest</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| style="background-color: #D9D9D9; width: 10%; "| | | style="background-color: #D9D9D9; width: 10%; "| | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Monster</b><BR><b>materiaal</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Afname</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>TestNaam</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Uitslag</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Referentie</b><BR><b>Ondergrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Referentie</b><BR><b>Bovengrens</b> | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Resultaat</b><BR><b>Vlaggen</b> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Klinische chemie | | style="width: 11%; "|Klinische chemie | ||
| Regel 485: | Regel 523: | ||
{|class="wikitable" width="1021px" style= "font-size: 9.5pt;" | {|class="wikitable" width="1021px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
|colspan="10" style="background-color: #1F497D; width: 100%; color | |colspan="10" style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>LaboratoriumUitslag</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Type</b></font> | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>Resultaat</b><BR></font><font color=#FFFFFF><b>Status</b></font> | ||
|colspan="2" style="background-color: #548DD4; width: 19%; color | |colspan="2" style="background-color: #548DD4; width: 19%; "|<font color=#FFFFFF><b>Monster</b></font> | ||
|colspan="6" style="background-color: #548DD4; width: 60%; color | |colspan="6" style="background-color: #548DD4; width: 60%; "|<font color=#FFFFFF><b>LaboratoriumTest</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 11%; "| | | style="background-color: #D9D9D9; width: 11%; "| | ||
| style="background-color: #D9D9D9; width: 10%; "| | | style="background-color: #D9D9D9; width: 10%; "| | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Monster</b><BR><b>materiaal</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Afname</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>TestNaam</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Test</b><BR><b>DatumTijd</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Uitslag</b> | ||
| style="background-color: #D9D9D9; width: 10% | | style="background-color: #D9D9D9; width: 10%; "|<b>Referentie</b><BR><b>Ondergrens</b> | ||
| style="background-color: #D9D9D9; width: 11% | | style="background-color: #D9D9D9; width: 11%; "|<b>Referentie</b><BR><b>Bovengrens</b> | ||
| style="background-color: #D9D9D9; width: 9% | | style="background-color: #D9D9D9; width: 9%; "|<b>Resultaat</b><BR><b>Vlaggen</b> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 11%; "|Virologie | | style="width: 11%; "|Virologie | ||
| Regel 522: | Regel 560: | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: <17636008 <noWiki>|</noWiki> specimen collection <noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=17636008 17636008]<noWiki>|</noWiki>specimen collection<noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
|} | |} | ||
=== ResultFlagsCodelist=== | === ResultFlagsCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Conceptcode | |Conceptcode<!--vsConceptCode--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|Description | |Description<!--vsDescription--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|High | |High | ||
| Regel 576: | Regel 615: | ||
|Sensitief | |Sensitief | ||
|} | |} | ||
=== ResultStatusCodelist=== | === ResultStatusCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Conceptcode | |Conceptcode<!--vsConceptCode--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|Description | |Description<!--vsDescription--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Pending | |Pending | ||
| Regel 619: | Regel 658: | ||
|Gecorrigeerd | |Gecorrigeerd | ||
|} | |} | ||
=== ResultTypeCodelist=== | === ResultTypeCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Conceptcode | |Conceptcode<!--vsConceptCode--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|Description | |Description<!--vsDescription--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Hematology | |Hematology | ||
|252275004 | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=252275004 252275004] | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 639: | Regel 678: | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Chemistry | |Chemistry | ||
|275711006 | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=275711006 275711006] | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 645: | Regel 684: | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Serology | |Serology | ||
|68793005 | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=68793005 68793005] | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 651: | Regel 690: | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Virology | |Virology | ||
|395124008 | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=395124008 395124008] | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 657: | Regel 696: | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Toxicology | |Toxicology | ||
|314076009 | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=314076009 314076009] | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
| Regel 663: | Regel 702: | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Microbiology | |Microbiology | ||
|19851009 | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=19851009 19851009] | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
|Microbiologie | |Microbiologie | ||
|} | |} | ||
=== SpecimenMaterialCodelist=== | === SpecimenMaterialCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|SNOMED CT: <123038009 <noWiki>|</noWiki> specimen <noWiki>|</noWiki> | |SNOMED CT: <[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=123038009 123038009]<noWiki>|</noWiki>specimen<noWiki>|</noWiki> | ||
|SNOMED CT | |SNOMED CT | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
|} | |} | ||
=== TestCodelist=== | === TestCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Alle waarden | |Alle waarden | ||
| Regel 698: | Regel 737: | ||
|2.16.840.1.113883.6.1 | |2.16.840.1.113883.6.1 | ||
|} | |} | ||
=== TestMethodCodelist=== | === TestMethodCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Alle waarden | |Alle waarden | ||
| Regel 713: | Regel 752: | ||
|2.16.840.1.113883.6.96 | |2.16.840.1.113883.6.96 | ||
|} | |} | ||
=== TestNameCodelist=== | === TestNameCodelist=== | ||
{| class="wikitable" width="60%" | {| class="wikitable" width="60%" | ||
|-style='vertical-align:top; background-color: #E8D7BE;' | |-style='vertical-align:top; background-color: #E8D7BE;' | ||
|Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 | |Valueset OID<!--vsValuesetOID-->: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 | ||
|Binding<!--vsBindingTag-->: | |||
|} | |} | ||
{| class="wikitable" width="60% " | {| class="wikitable" width="60% " | ||
|-style="background-color: #1F497D; color: white; font-weight: bold; " | |-style="background-color: #1F497D; color: white; font-weight: bold; " | ||
|Conceptname | |Conceptname<!--vsConceptName--> | ||
|Codesystem name||Codesystem OID | |Codesystem name<!--vsConceptSystemName-->||Codesystem OID<!--vsConceptSystemOID--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|Alle waarden | |Alle waarden | ||
| Regel 729: | Regel 768: | ||
|} | |} | ||
==This information model in other releases<!--ftOtherReleases-->== | |||
== | <!-- Hieronder wordt een transclude page aangeroepen --> | ||
{{Versions-2.16.840.1.113883.2.4.3.11.60.40.3.13.1(EN)|2|2016}} | |||
This information model is also available as [[Media:nl.zorg.LaboratoryTestResultForTransfer-v3.0(2016EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.LaboratoryTestResultForTransfer-v3.0(2016EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | <!-- Tot hier de transclude page --> | ||
==About this information== | ==Information model references<!--ftReferences-->== | ||
The information in this wikipage is based on Release summer 2016 <BR> | ====This information model refers to<!--ftRefersTo-->==== | ||
Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | :-- | ||
This page is generated on | ====This information model is used in<!--ftReferredBy-->==== | ||
:-- | |||
==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | |||
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | |||
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:<!--ftReferenceIntro--> | |||
<ul> | |||
<li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment<!--ftArtDecorReference--> [http://decor.nictiz.nl/art-decor/decor-scenarios--zib2016bbr-?id=2.16.840.1.113883.2.4.3.11.60.6.4.2.13.1&effectiveDate=2016-05-01T00:00:00&language=en-US&scenariotree=false [[File:artdecor.jpg|16px|link=]]]</li> | |||
<li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR<!--ftSimplifierReference--> {{SimplefierLinks|2016}}</li> | |||
</ul> | |||
==Downloads<!--ftDownloadTitle-->== | |||
This information model is also available as [[Media:nl.zorg.LaboratoryTestResultForTransfer-v3.0(2016EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.LaboratoryTestResultForTransfer-v3.0(2016EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]]<!--ftDownloads--> | |||
==About this information<!--ftHeader2-->== | |||
The information in this wikipage is based on<!--ftInfoBase--> Release summer 2016 <BR> | |||
Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | |||
This page is generated on 21/12/2018 15:40:38 with ZibExtraction v. 3.0.6929.24609<!--ftDate--> <BR> | |||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2016(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2016(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | ||
Huidige versie van 24 dec 2018 om 12:09
General information
Name: nl.zorg.LaboratoryTestResultForTransfer ![]()
Version: 3.0
HCIM Status:Final
Release: 2016
Release status: Published
Release date: 1-5-2016
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 7-6-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.13.1 |
| DCM::KeywordList | laboratorium uitslag, lab, laboratorium bepaling |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.OverdrachtLaboratoriumUitslag |
| DCM::PublicationDate | 1-5-2016 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 25-8-2015 |
| DCM::Superseeds | nl.nfu.OverdrachtLaboratoriumUitslag-v1.2.2 |
| DCM::Version | 3.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-238 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept TestNaam opsplitsen. |
| ZIB-239 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet SNOMED - eLab valueset van concept Testmethode opsplitsen. |
| ZIB-240 | In de klinische bouwsteen OverdrachtLabUitslag kwam de tagged value DCM::ValueSet van concept LaboratoriumTest niet overeen met de naam van de gekoppelde waardenlijst ResultNormalcyStatus Valueset (HL7). |
| ZIB-241 | In de klinische bouwsteen OverdrachtLabUitslag de tagged value DCM::ValueSet LOINC - eLab valueset van concept Onderzoek opsplitsen. |
| ZIB-242 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatStatus niet overeen met de tagged value van het concept. |
| ZIB-243 | In de klinische bouwsteen OverdrachtLabuitslag kwam de naam van de gekoppelde waardenlijst van concept ResultaatType niet overeen met de tagged value van het concept. |
| ZIB-244 | Tagged values van concept Onderzoek van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-245 | Tagged values van concept Testmethode van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-246 | Tagged values van concept TestNaam van OverdrachtLabUitslag aangepast, door tagged value DCM:ValueSet e-lab een codelijst naam te geven incl. in tagged value notes verwijzing naar extern codesystem. |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
| ZIB-361 | Naamgeving concept Opmerking aangepast |
| ZIB-367 | Opschonen ResultaatVlaggenCodelijst |
| ZIB-370 | ResultaatStatusCodelijst en TekstUitslagCodelijst codes aanpassen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 1.2.1 (22-05-2015)
| ZIB-392 | De ResultaatTypeCodelijst heeft geen "OID: " aanduiding in de onderliggende codelijst. |
Publicatieversie 1.2.2 (16-07-2015)
| ZIB-420 | Vervallen SNOMED CT code in ResultaatTypeCodelijst |
Publicatieversie 3.0 (01-05-2016)
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
.
Concept
A laboratory result describes the result of a laboratory analysis.
In addition to the results of tests with a singular result, the results of more complex tests with multiple results or a ‘panel’ can also be recorded.
Purpose
Laboratory tests are done for the purpose of diagnosing and preventing disease and follow-up on the effects of treatment.
Evidence Base
There are two information models for recording laboratory test results: TextResultTransfer and LaboratoryResultTransfer.
In the case of laboratory test results, it is difficult to clearly indicate exactly when to use this information model and when to use the TextResultTransfer information model.
In general, laboratory tests resulting in a value (7.1 mmol/L), ordinal number (++ from series to ++++) or a quantitative result (Low) are recorded using this information model. The TextResultTransfer information model is better suited for textual results that are more descriptive in nature and which are longer than just a few words. Both types of tests occur in almost all laboratories.
The applicability of the aforementioned information models is not determined by the kind of lab but by the kind of result.
In developing the information model, the definitions were used from the data set and coding choices from the IHE/Nictiz e-Lab program.
The now determined information model is a subset of the e-Lab data set, provided that the detailing that is less relevant to the general transfer use case was left out. If this information is required, it can be entered in the comments field.
Information Model
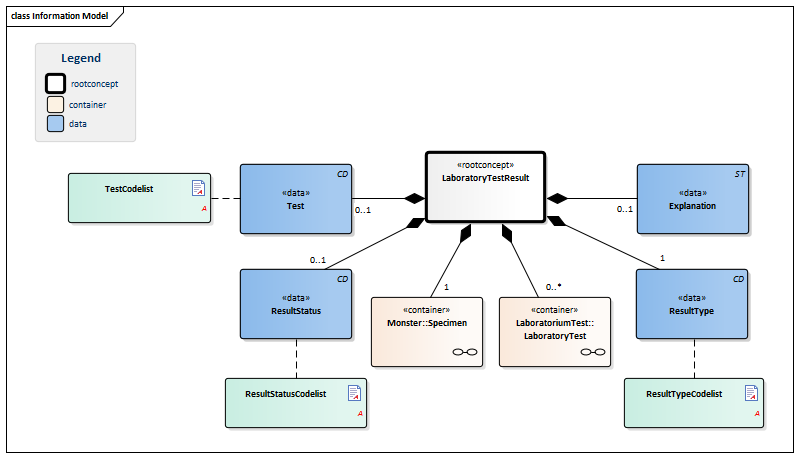
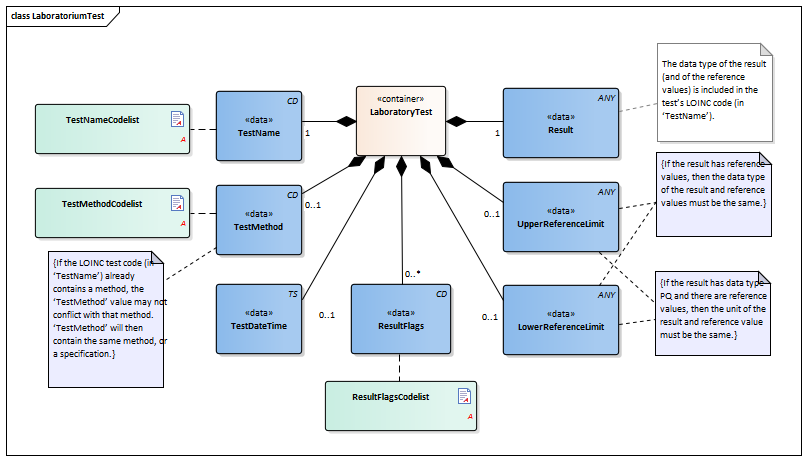
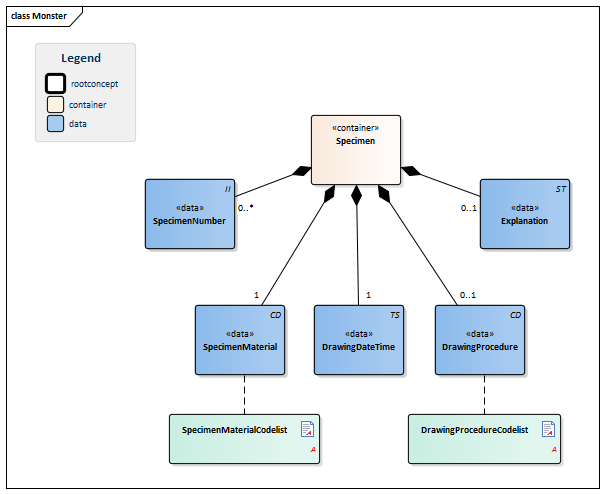
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:13.1.1 | Root concept of the LaboratoryTestResultTransfer information model. This root concept contains all data elements of the Laboratory TestResultTransfer information model. | ||||||||||||
| NL-CM:13.1.2 | 1 | Container of the Specimen concept. This container contains all data elements of the Specimen concept. | |||||||||||
| NL-CM:13.1.15 | 0..* | Identification number of the material obtained, as a reference for inquiries to the source organization. In a transmural setting, this number will consist of a specimen number including the identification of the issuing organization, to be unique outside of the borders of an organization. | |||||||||||
| NL-CM:13.1.16 | 1 | SpecimenMaterial describes the material obtained. If the LOINC test code also implicitly describes a material, this element may not conflict with the description. If desired, this component can provide a more detailed description of the material: LOINC codes only contain the materials at a main level.
This is in line with the agreements made in the IHE/Nictiz program e-Lab. If the test is carried out on derived material (such as plasma), this element will still contain the material drawn (in this case, blood). In this case, the LOINC code will generally refer to plasma. |
| ||||||||||
| NL-CM:13.1.17 | 1 | Time at which the material was drawn. |
|
||||||||||
| NL-CM:13.1.18 | 0..1 | If relevant for the results, the method of obtaining the specimen can be entered as well. |
| ||||||||||
| NL-CM:13.1.19 | 0..1 | Comments on administering the test, such as drawing material after a (glucose) stimulus or taking medicine. |
|
||||||||||
| NL-CM:13.1.3 | 0..* | Container of the LaboratoryTest concept. This container contains all data elements of the LaboratoryTest concept. | |||||||||||
| NL-CM:13.1.8 | 1 | The TestName is the name of the executed test. |
| ||||||||||
| NL-CM:13.1.9 | 0..1 | The test method used to obtain the result. |
| ||||||||||
| NL-CM:13.1.13 | 0..1 | The date and if possible the time at which the test was carried out. | |||||||||||
| NL-CM:13.1.10 | 1 | The test result. Depending on the type of test, the result will consist of a value with a unit or a coded value (ordinal or nominal). | |||||||||||
| NL-CM:13.1.11 | 0..1 | The upper reference limit for the patient of the value measured in the test. | |||||||||||
| NL-CM:13.1.12 | 0..1 | The lower reference limit for the patient of the value measured with the test. | |||||||||||
| NL-CM:13.1.14 | 0..* | Attention codes indicating whether the result is above or below certain reference values. |
| ||||||||||
| NL-CM:13.1.4 | 0..1 | For laboratory tests comprising multiple subtests and often requested together as a whole, this concept contains the name of the compound request (often indicated as a ‘panel’, ‘battery’ or ‘cluster’). Examples include: blood gases and EBV serology. |
| ||||||||||
| NL-CM:13.1.6 | 0..1 | The status of the laboratory test result. |
| ||||||||||
| NL-CM:13.1.5 | 0..1 | Comments, such as a textual interpretation or advice accompanying the result, for example. |
|
||||||||||
| NL-CM:13.1.7 | 1 | The type of result defines the laboratory specialty under which the test is categorized. |
| ||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestNaam | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Resultaat Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 12-06-2012 09:00 | Natrium | 12-06-2012 13:15 | 138 mmol/l | 136 mmol/l | 146 mmol/l | |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestNaam | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Resultaat Vlaggen | ||
| Klinische chemie | Definitief | Bloed | 23-05-2012 08:08 | Chloride | 23-05-2012 12:00 | 109 mmol/l | 99 mmol/l | 108 mmol/l | Boven referentie- waarde |
| LaboratoriumUitslag | |||||||||
| Resultaat Type |
Resultaat Status |
Monster | LaboratoriumTest | ||||||
| Monster materiaal |
Afname DatumTijd |
TestNaam | Test DatumTijd |
Uitslag | Referentie Ondergrens |
Referentie Bovengrens |
Resultaat Vlaggen | ||
| Virologie | Definitief | Bloed | 16-01-2012 08:00 | Hepatitis A IgM | 16-01-2012 10:12 | Negatief | |||
References
1. Nederlandse Vereniging voor Medische Microbiologie (2010) ELab en EvT. [Online] Beschikbaar op: http://www.nvmm.nl/ict/vereniging/werkgroepen_commissies/elab-en-evt [Geraadpleegd: 23 juli 2014].
Valuesets
DrawingProcedureCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.2 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <17636008|specimen collection| | SNOMED CT | 2.16.840.1.113883.6.96 |
ResultFlagsCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.7 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| High | H | ObservationInterpretation | 2.16.840.1.113883.5.83 | Boven referentiewaarde |
| Low | L | ObservationInterpretation | 2.16.840.1.113883.5.83 | Onder referentiewaarde |
| Intermediate | I | ObservationInterpretation | 2.16.840.1.113883.5.83 | Variabel |
| Resistant | R | ObservationInterpretation | 2.16.840.1.113883.5.83 | Resistent |
| Susceptible | S | ObservationInterpretation | 2.16.840.1.113883.5.83 | Sensitief |
ResultStatusCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.8 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Pending | pending | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Uitslag volgt |
| Preliminary | preliminary | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Voorlopig |
| Final | final | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Definitief |
| Appended | appended | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Aanvullend |
| Corrected | corrected | ResultaatStatus | 2.16.840.1.113883.2.4.3.11.60.40.4.16.1 | Gecorrigeerd |
ResultTypeCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.1 | Binding: |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Hematology | 252275004 | SNOMED CT | 2.16.840.1.113883.6.96 | Hematologie |
| Chemistry | 275711006 | SNOMED CT | 2.16.840.1.113883.6.96 | Klinische chemie |
| Serology | 68793005 | SNOMED CT | 2.16.840.1.113883.6.96 | Serologie/ immunologie |
| Virology | 395124008 | SNOMED CT | 2.16.840.1.113883.6.96 | Virologie |
| Toxicology | 314076009 | SNOMED CT | 2.16.840.1.113883.6.96 | Toxicologie |
| Microbiology | 19851009 | SNOMED CT | 2.16.840.1.113883.6.96 | Microbiologie |
SpecimenMaterialCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.6 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <123038009|specimen| | SNOMED CT | 2.16.840.1.113883.6.96 |
TestCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.5 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
TestMethodCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.4 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | SNOMED CT | 2.16.840.1.113883.6.96 |
TestNameCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.13.1.3 | Binding: |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | LOINC | 2.16.840.1.113883.6.1 |
This information model in other releases
- Release 2015, (Version 1.2.2)
- Release 2017, (Version 4.1)
- Prerelease 2018-2, (Version 4.3)
- Prerelease 2019-2, (Version 4.5)
- Release 2020, (Version 4.6)
- Prerelease 2021-2, (Version 5.0)
- Prerelease 2022-1, (Version 5.1)
- Prerelease 2023-1, (Version 6.0)
- Prerelease 2024-1, (Versie 7.0)
- Release 2024, (Version 7.1)
Information model references
This information model refers to
- --
This information model is used in
- --
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Release summer 2016
Conditions for use are located on the mainpage ![]()
This page is generated on 21/12/2018 15:40:38 with ZibExtraction v. 3.0.6929.24609