MedicalDevice-v1.2(2015EN): verschil tussen versies
(Nieuwe pagina aangemaakt met '==General information== Name: '''nl.nfu.MedicalDevice''' link=MedischHulpmiddel-v1.2(2015NL)<BR> Version: '''1.2''' <br> HCIM Status:Final<br>...') |
Geen bewerkingssamenvatting |
||
| Regel 3: | Regel 3: | ||
Version: '''1.2''' <br> | Version: '''1.2''' <br> | ||
HCIM Status:Final<br> | HCIM Status:Final<br> | ||
Release: '''2015''' <br> | |||
Release status: Published<br> | Release status: Published<br> | ||
Release date: 1-4-2015 | |||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | ||
| Regel 153: | Regel 153: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
<imagemap> Bestand:MedicalDevice-v1.2Model(EN).png | center | |||
rect 156 275 281 345 [[#9809]] | |||
rect 350 372 464 442 [[HealthProfessional-v1.2.1(2015EN)]] | |||
rect 156 372 281 442 [[HealthcareProvider-v1.2(2015EN)]] | |||
rect 514 316 676 386 [[#9810]] | |||
rect 156 178 281 248 [[ConcernForTransfer-v1.2(2015EN)]] | |||
rect 654 208 744 278 [[#9806]] | |||
rect 653 106 743 176 [[#9805]] | |||
rect 516 157 606 227 [[#9804]] | |||
rect 156 81 281 151 [[#9807]] | |||
rect 358 153 462 233 [[#9803]] | |||
rect 785 113 914 163 [[#ProductIDCodelist]] | |||
rect 485 437 705 487 [[#AidAnatomicalLocationCodelist]] | |||
rect 785 217 914 267 [[#ProductTypeCodelist]] | |||
desc none | |||
</imagemap> | |||
<BR> | <BR> | ||
{| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | {| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | ||
| Regel 159: | Regel 174: | ||
|style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | |style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | ||
|-style="vertical-align:top; background-color: #E3E3E3; " | |-style="vertical-align:top; background-color: #E3E3E3; " | ||
|style = "text-align:center" |[[Bestand: block.png| 20px]] | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | ||
||NL-CM:10.1.1 | ||NL-CM:10.1.1 | ||
|colspan ="6" style ="padding-left: 0px"|<span Title="NL: MedischHulpmiddel">[[Bestand: arrowdown.png | 10px]]MedicalAid</span> | |colspan ="6" style ="padding-left: 0px"|<span Id=9803 Title="NL: MedischHulpmiddel">[[Bestand: arrowdown.png | 10px | link=]]MedicalAid</span> | ||
| | | | ||
|Root concept of the MedicalAid building block. This root concept contains all data elements of the MedicalAid building block. | |Root concept of the MedicalAid building block. This root concept contains all data elements of the MedicalAid building block. | ||
| Regel 167: | Regel 182: | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:10.1.2 | ||NL-CM:10.1.2 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Product">[[Bestand: arrowdown.png | 10px]]Product</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9804 Title="NL: Product">[[Bestand: arrowdown.png | 10px | link=]]Product</span> | ||
|1 | |1 | ||
|The medical aid used (internally or externally). | |The medical aid used (internally or externally). | ||
| Regel 176: | Regel 191: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:10.1.4 | ||NL-CM:10.1.4 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ProductID">[[Bestand: arrowright.png | 10px]]ProductID</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=9805 Title="NL: ProductID">[[Bestand: arrowright.png | 10px | link=]]ProductID</span> | ||
|0..1 | |0..1 | ||
|Unique identification of the product, such as the serial number. | |Unique identification of the product, such as the serial number. | ||
| Regel 192: | Regel 207: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductIDCodelist|ProductIDCodelist]] | |[[Bestand: List2.png | link=#ProductIDCodelist]]||[[#ProductIDCodelist|ProductIDCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:10.1.3 | ||NL-CM:10.1.3 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ProductType">[[Bestand: arrowright.png | 10px]]ProductType</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=9806 Title="NL: ProductType">[[Bestand: arrowright.png | 10px | link=]]ProductType</span> | ||
|0..1 | |0..1 | ||
|The code of the type of product. | |The code of the type of product. | ||
| Regel 206: | Regel 221: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductTypeCodelist|ProductTypeCodelist]] | |[[Bestand: List2.png | link=#ProductTypeCodelist]]||[[#ProductTypeCodelist|ProductTypeCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:10.1.11 | ||NL-CM:10.1.11 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: BeginDatum">[[Bestand: arrowright.png | 10px]]StartDate</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9807 Title="NL: BeginDatum">[[Bestand: arrowright.png | 10px | link=]]StartDate</span> | ||
|0..1 | |0..1 | ||
|The start date of the first use or implant of the medical aid. A ‘vague’ date, such as only the year, is permitted. | |The start date of the first use or implant of the medical aid. A ‘vague’ date, such as only the year, is permitted. | ||
| Regel 218: | Regel 233: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] | ||
||NL-CM:10.1.7 | ||NL-CM:10.1.7 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Indicatie::Probleem">[[Bestand: arrowright.png | 10px]]Indication::Problem</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9808 Title="NL: Indicatie::Probleem">[[Bestand: arrowright.png | 10px | link=]]Indication::Problem</span> | ||
|0..* | |0..* | ||
|The medical reason for use of the medical aid. | |The medical reason for use of the medical aid. | ||
| Regel 228: | Regel 243: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: block.png]]||[[ConcernForTransfer-v1.2(2015EN) |ConcernForTransfer]] | |[[Bestand: block.png | link=ConcernForTransfer-v1.2(2015EN)]]||[[ConcernForTransfer-v1.2(2015EN) |ConcernForTransfer]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:10.1.10 | ||NL-CM:10.1.10 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Explanation</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9809 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Explanation</span> | ||
|0..1 | |0..1 | ||
|Comment about use or information on the medical aid used. | |Comment about use or information on the medical aid used. | ||
| Regel 240: | Regel 255: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:10.1.6 | ||NL-CM:10.1.6 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: HulpmiddelAnatomischeLocatie">[[Bestand: arrowright.png | 10px]]AidAnatomicalLocation</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9810 Title="NL: HulpmiddelAnatomischeLocatie">[[Bestand: arrowright.png | 10px | link=]]AidAnatomicalLocation</span> | ||
|0..1 | |0..1 | ||
|Patient’s anatomical location of the medical aid used. | |Patient’s anatomical location of the medical aid used. | ||
| Regel 250: | Regel 265: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#AidAnatomicalLocationCodelist|AidAnatomicalLocationCodelist]] | |[[Bestand: List2.png | link=#AidAnatomicalLocationCodelist]]||[[#AidAnatomicalLocationCodelist|AidAnatomicalLocationCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] | ||
||NL-CM:10.1.8 | ||NL-CM:10.1.8 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Locatie::Zorgaanbieder">[[Bestand: arrowright.png | 10px]]Location::HealthcareProvider</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9811 Title="NL: Locatie::Zorgaanbieder">[[Bestand: arrowright.png | 10px | link=]]Location::HealthcareProvider</span> | ||
|0..1 | |0..1 | ||
|The healthcare provider at which use of the medical aid was initiated or where the aid was implanted. | |The healthcare provider at which use of the medical aid was initiated or where the aid was implanted. | ||
| Regel 263: | Regel 278: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: block.png]]||[[HealthcareProvider-v1.2(2015EN) |HealthcareProvider]] | |[[Bestand: block.png | link=HealthcareProvider-v1.2(2015EN)]]||[[HealthcareProvider-v1.2(2015EN) |HealthcareProvider]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] | ||
||NL-CM:10.1.9 | ||NL-CM:10.1.9 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Zorgverlener">[[Bestand: arrowright.png | 10px]]HealthcareProvider</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9812 Title="NL: Zorgverlener">[[Bestand: arrowright.png | 10px | link=]]HealthcareProvider</span> | ||
|0..1 | |0..1 | ||
|The healthcare provider involved in the indication for use of the medical aid implant. | |The healthcare provider involved in the indication for use of the medical aid implant. | ||
| Regel 276: | Regel 291: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: block.png]]||[[HealthProfessional-v1.2.1(2015EN) |HealthProfessional]] | |[[Bestand: block.png | link=HealthProfessional-v1.2.1(2015EN)]]||[[HealthProfessional-v1.2.1(2015EN) |HealthProfessional]] | ||
|} | |} | ||
|} | |} | ||
| Regel 287: | Regel 302: | ||
{|class="wikitable" width="997px" style= "font-size: 9.5pt;" | {|class="wikitable" width="997px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #1F497D; width: 10%; color | | style="background-color: #1F497D; width: 10%; "|<font color=#FFFFFF><b>Begin</b><BR></font><font color=#FFFFFF><b>D</b></font><font color=#FFFFFF><b>atum</b></font> | ||
| style="background-color: #1F497D; width: 10%; color | | style="background-color: #1F497D; width: 10%; "|<font color=#FFFFFF><b>Product</b></font> | ||
| style="background-color: #1F497D; width: 15%; "| | | style="background-color: #1F497D; width: 15%; "| | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Hulpmiddel Anatomische Locatie</b></font> | ||
| style="background-color: #1F497D; width: 16%; color | | style="background-color: #1F497D; width: 16%; "|<font color=#FFFFFF><b>Indicatie</b></font> | ||
| style="background-color: #1F497D; width: 11%; color | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>Locatie</b></font> | ||
| style="background-color: #1F497D; width: 12%; "| | | style="background-color: #1F497D; width: 12%; "| | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Toelichting</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 10%; "| | | style="background-color: #548DD4; width: 10%; "| | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>ProductID</b></font> | ||
| style="background-color: #548DD4; width: 15%; color | | style="background-color: #548DD4; width: 15%; "|<font color=#FFFFFF><b>ProductType</b></font> | ||
| style="background-color: #548DD4; width: 13%; "| | | style="background-color: #548DD4; width: 13%; "| | ||
| style="background-color: #548DD4; width: 16%; color | | style="background-color: #548DD4; width: 16%; "|<font color=#FFFFFF><b>ProbleemNaam</b></font> | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Organisatie</b><BR></font><font color=#FFFFFF><b>Naam</b></font> | ||
| style="background-color: #548DD4; width: 12%; color | | style="background-color: #548DD4; width: 12%; "|<font color=#FFFFFF><b>Afdeling</b><BR></font><font color=#FFFFFF><b>Specialisme</b></font> | ||
| style="background-color: #548DD4; width: 13%; "| | | style="background-color: #548DD4; width: 13%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| Regel 323: | Regel 338: | ||
| style="width: 13%; "| | | style="width: 13%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #1F497D; width: 10%; color | | style="background-color: #1F497D; width: 10%; "|<font color=#FFFFFF><b>Begin</b><BR></font><font color=#FFFFFF><b>D</b></font><font color=#FFFFFF><b>atum</b></font> | ||
| style="background-color: #1F497D; width: 10%; color | | style="background-color: #1F497D; width: 10%; "|<font color=#FFFFFF><b>Product</b></font> | ||
| style="background-color: #1F497D; width: 15%; "| | | style="background-color: #1F497D; width: 15%; "| | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Hulpmiddel Anatomische Locatie</b></font> | ||
| style="background-color: #1F497D; width: 16%; color | | style="background-color: #1F497D; width: 16%; "|<font color=#FFFFFF><b>Indicatie</b></font> | ||
| style="background-color: #1F497D; width: 11%; color | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>Locatie</b></font> | ||
| style="background-color: #1F497D; width: 12%; "| | | style="background-color: #1F497D; width: 12%; "| | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Toelichting</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 10%; "| | | style="background-color: #548DD4; width: 10%; "| | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>ProductID</b></font> | ||
| style="background-color: #548DD4; width: 15%; color | | style="background-color: #548DD4; width: 15%; "|<font color=#FFFFFF><b>ProductType</b></font> | ||
| style="background-color: #548DD4; width: 13%; "| | | style="background-color: #548DD4; width: 13%; "| | ||
| style="background-color: #548DD4; width: 16%; color | | style="background-color: #548DD4; width: 16%; "|<font color=#FFFFFF><b>ProbleemNaam</b></font> | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Organisatie</b><BR></font><font color=#FFFFFF><b>Naam</b></font> | ||
| style="background-color: #548DD4; width: 12%; color | | style="background-color: #548DD4; width: 12%; "|<font color=#FFFFFF><b>Afdeling</b><BR></font><font color=#FFFFFF><b>Specialisme</b></font> | ||
| style="background-color: #548DD4; width: 13%; "| | | style="background-color: #548DD4; width: 13%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| Regel 359: | Regel 374: | ||
| style="width: 13%; "| | | style="width: 13%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #1F497D; width: 10%; color | | style="background-color: #1F497D; width: 10%; "|<font color=#FFFFFF><b>Begin</b><BR></font><font color=#FFFFFF><b>D</b></font><font color=#FFFFFF><b>atum</b></font> | ||
| style="background-color: #1F497D; width: 10%; color | | style="background-color: #1F497D; width: 10%; "|<font color=#FFFFFF><b>Product</b></font> | ||
| style="background-color: #1F497D; width: 15%; "| | | style="background-color: #1F497D; width: 15%; "| | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Hulpmiddel Anatomische Locatie</b></font> | ||
| style="background-color: #1F497D; width: 16%; color | | style="background-color: #1F497D; width: 16%; "|<font color=#FFFFFF><b>Indicatie</b></font> | ||
| style="background-color: #1F497D; width: 11%; color | | style="background-color: #1F497D; width: 11%; "|<font color=#FFFFFF><b>Locatie</b></font> | ||
| style="background-color: #1F497D; width: 12%; "| | | style="background-color: #1F497D; width: 12%; "| | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Toelichting</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 10%; "| | | style="background-color: #548DD4; width: 10%; "| | ||
| style="background-color: #548DD4; width: 10%; color | | style="background-color: #548DD4; width: 10%; "|<font color=#FFFFFF><b>ProductID</b></font> | ||
| style="background-color: #548DD4; width: 15%; color | | style="background-color: #548DD4; width: 15%; "|<font color=#FFFFFF><b>ProductType</b></font> | ||
| style="background-color: #548DD4; width: 13%; "| | | style="background-color: #548DD4; width: 13%; "| | ||
| style="background-color: #548DD4; width: 16%; color | | style="background-color: #548DD4; width: 16%; "|<font color=#FFFFFF><b>ProbleemNaam</b></font> | ||
| style="background-color: #548DD4; width: 11%; color | | style="background-color: #548DD4; width: 11%; "|<font color=#FFFFFF><b>Organisatie</b><BR></font><font color=#FFFFFF><b>Naam</b></font> | ||
| style="background-color: #548DD4; width: 12%; color | | style="background-color: #548DD4; width: 12%; "|<font color=#FFFFFF><b>Afdeling</b><BR></font><font color=#FFFFFF><b>Specialisme</b></font> | ||
| style="background-color: #548DD4; width: 13%; "| | | style="background-color: #548DD4; width: 13%; "| | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| Regel 440: | Regel 455: | ||
==This information model in other releases== | |||
<ul> | |||
<li>[[MedicalDevice-v3.0(2016EN) | Release 2016, (Version 3.0)]]</li> | |||
<li>[[MedicalDevice-v3.1(2017EN) | Release 2017, (Version 3.1)]]</li> | |||
</ul> | |||
==More on this information model== | ==More on this information model== | ||
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | |||
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications: | |||
<ul> | |||
<li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment [http://decor.nictiz.nl/art-decor/decor-scenarios--zib2015bbr-?id=2.16.840.1.113883.2.4.3.11.60.5.4.2.10.1&effectiveDate=2015-04-01T00:00:00&language=en-US&scenariotree=false [[File:artdecor.jpg|16px|link=]]]</li> | |||
<li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR [https://simplifier.net/NictizSTU3/~resources?text=zib&category=StructureDefinition [[File:fhir.png|link=]]]</li> | |||
</ul> | |||
This information model is also available as [[Media:nl.nfu.MedicalDevice-v1.2(2015EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.nfu.MedicalDevice-v1.2(2015EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | This information model is also available as [[Media:nl.nfu.MedicalDevice-v1.2(2015EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.nfu.MedicalDevice-v1.2(2015EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | ||
==About this information== | ==About this information== | ||
The information in this wikipage is based on ''Registratie aan de bron'' publication 2015 including errata dd. 16-07-2015 <BR> | The information in this wikipage is based on ''Registratie aan de bron'' publication 2015 including errata dd. 16-07-2015 <BR> | ||
Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | ||
This page is generated on | This page is generated on 24/01/2018 17:13:11 <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | ||
Versie van 24 jan 2018 23:20
General information
Name: nl.nfu.MedicalDevice ![]()
Version: 1.2
HCIM Status:Final
Release: 2015
Release status: Published
Release date: 1-4-2015
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 2-1-2013 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | NFU |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.10.1 |
| DCM::KeywordList | medisch hulpmiddel, implantaat |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.nfu.MedischHulpmiddel |
| DCM::PublicationDate | 1-4-2015 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 1-4-2015 |
| DCM::Superseeds | |
| DCM::Version | 1.2 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013) -
Publicatieversie 1.1 (01-07-2013)
| ZIB-11 | Gebruik van de GS1 standaard |
Publicatieversie 1.2 (01-04-2015)
| ZIB-83 | Wijzigingsvoorstel OverdrachtMedischHulpmiddel |
| ZIB-88 | OverdrachtMedischHulpmiddel |
| ZIB-110 | Example of the instrument van OverdrachtMedischHulpmiddel bevat kolom Zorgverlener, definities binnen deze kolom moeten duidelijker |
| ZIB-249 | In de klinische bouwsteen OverdrachtMedischHulpmiddel DCM::ValueSet SNOMED CT aangepast van concept AnatomischeLocalisatie naar tagged value DCM::ContentExpression. |
| ZIB-250 | In klinische bouwsteen OverdrachtMedischHulpmiddel de waarde van DCM::Name aanpassen van OverdrachtMedischHulpmiddel naar nl.nfu.OverdrachtMedischHulpmiddel. |
| ZIB-251 | Tagged value DCM::ValueSet van concept ProductType aangepast naar DCM::CodeSystem. |
| ZIB-252 | Tagged value DCM::ValueSet GTIN aangepast naar DCM::AssigningAuthority GTIN. |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
| ZIB-327 | AnatomischeLocalisatie wijzigen in AnatomischeLocatie |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Concept
Medical aids are any internally implanted and external devices and/or aids used by the patient (in the past) to reduce the effects of functional limitations in organ systems or to facilitate the treatment of a disease.
Purpose
Data on medical aids is recorded for several reasons. Knowledge of the presence of these implants enables tracing and taking the aid or device into account in diagnostic or therapeutic procedures, care and transport.
Examples include:
- Consequences for transportation, toilet use, etc., in the case of a wheelchair;
- A pacemaker can be of medical importance, but also has consequences for planning radiological exams.
Evidence Base
Recording data on medically complex devices such as pacemakers is not yet common in EPD systems in the Netherlands, but is sometimes lacking: a letter from a specialist for example often does not include information on which type of pacemaker the patient has (and from which manufacturer).
The NFU opts for GS1 standards to increase patient safety and improve logistic efficiency.
The Dutch Ministry of Health, Welfare and Sport (VWS) will pass legislation for a national basic register of implants. Every healthcare center will have to supply a UDI (Unique Device Identification, with a link to GTIN) and a UPI (Unique Patient Identification) to the basic register. This will prevent situations in which a large group of patients have an aid or implant in which problems have been detected that cannot be traced.
Information Model
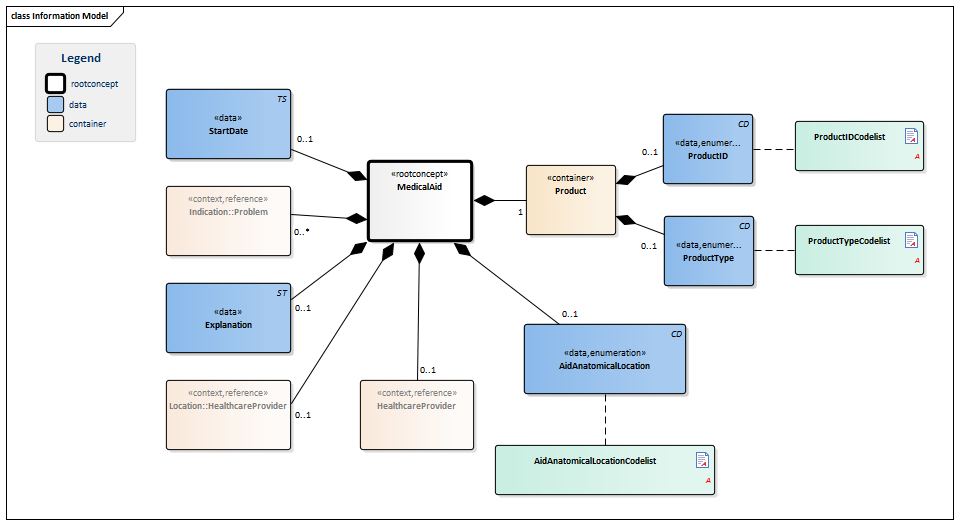
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:10.1.1 | Root concept of the MedicalAid building block. This root concept contains all data elements of the MedicalAid building block. | ||||||||||||
| NL-CM:10.1.2 | 1 | The medical aid used (internally or externally). | |||||||||||
| NL-CM:10.1.4 | 0..1 | Unique identification of the product, such as the serial number.
If the law requires this to be registered on the basis of a UDI (Unique Device Identifier), the unique identification must consist of a UDI-DI (Device Identifier) and a UDI-PI (Production Identifier(s)). See http://www.gs1.org/healthcare/udi for more information. The UDI-DI must be recorded in reference to GS1 GTIN (01) encryptions, with which for example a firm is linked to the product type. The UDI-PI must consist of the following: application identifier (AI); expiration date (17) and serial number (21) and/or batch or lot number (10). |
| ||||||||||
| NL-CM:10.1.3 | 0..1 | The code of the type of product. |
| ||||||||||
| NL-CM:10.1.11 | 0..1 | The start date of the first use or implant of the medical aid. A ‘vague’ date, such as only the year, is permitted. | |||||||||||
| NL-CM:10.1.7 | 0..* | The medical reason for use of the medical aid. |
| ||||||||||
| NL-CM:10.1.10 | 0..1 | Comment about use or information on the medical aid used. | |||||||||||
| NL-CM:10.1.6 | 0..1 | Patient’s anatomical location of the medical aid used. |
| ||||||||||
| NL-CM:10.1.8 | 0..1 | The healthcare provider at which use of the medical aid was initiated or where the aid was implanted. |
| ||||||||||
| NL-CM:10.1.9 | 0..1 | The healthcare provider involved in the indication for use of the medical aid implant. |
| ||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| Begin Datum |
Product | Hulpmiddel Anatomische Locatie | Indicatie | Locatie | Toelichting | ||
| ProductID | ProductType | ProbleemNaam | Organisatie Naam |
Afdeling Specialisme |
|||
| 08-03-2012 | 42192210 | Rolstoel | Multiple sclerose | Kan korte afstanden lopen | |||
| Begin Datum |
Product | Hulpmiddel Anatomische Locatie | Indicatie | Locatie | Toelichting | ||
| ProductID | ProductType | ProbleemNaam | Organisatie Naam |
Afdeling Specialisme |
|||
| 2007 | 42144000 | Gehoorapparaat | R oor | Presbyacusis | St. Franciscus Gasthuis | Audiologie | Apparaat niet zichtbaar (diep in de gehooringang) |
| Begin Datum |
Product | Hulpmiddel Anatomische Locatie | Indicatie | Locatie | Toelichting | ||
| ProductID | ProductType | ProbleemNaam | Organisatie Naam |
Afdeling Specialisme |
|||
| 10-02-2004 | 422033500 | VVI Pacemaker | L subclavian pouch | Paroxymaal boezemfibrilleren | Academisch Medisch Centrum | Cardiologie | Laatst doorgemeten in 2011 |
Issues
The UNSPSC code system has a great many products (including non-medical products). That is why a Dutch set and/or subcollection of this code system is required to indicate the type of product. We have currently opted to consider all values in the UNPSPSC for documenting the type of medical aid product in the absence of such a set.
References
1. Kamerbrief over het voorstel voor een register van implantaten. [Online] Beschikbaar op: http://www.rijksoverheid.nl/documenten-en-publicaties/kamerstukken/2012/11/20/kamerbrief-over-het-voorstel-voor-een-register-van-implantaten.html [Geraadpleegd: 15 september 2014].
Valuesets
AidAnatomicalLocationCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.2 |
| Conceptname | Codesystem name | Codesystem OID |
| SNOMED CT: <<91723000 | anatomical structure | | SNOMED CT | 2.16.840.1.113883.6.96 |
ProductIDCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.3 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Global Trade Item Number (GTIN) | 1.3.160 |
ProductTypeCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.10.1.1 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | UNSPSC | 2.16.840.1.113883.6.302 |
This information model in other releases
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Registratie aan de bron publication 2015 including errata dd. 16-07-2015
Conditions for use are located on the mainpage ![]()
This page is generated on 24/01/2018 17:13:11