Vaccination-v4.0(2019EN)
General information
Name: nl.zorg.Vaccination ![]()
Version: 4.0
HCIM Status:Final
Release: 2019
Release status: Prepublished
Release date: 06-07-2019
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 5-10-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.11.1 |
| DCM::KeywordList | vaccinatie, inenting |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.zorg.Vaccinatie |
| DCM::PublicationDate | 06-07-2019 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 01-07-2019 |
| DCM::Superseeds | nl.zorg.Vaccinatie-v3.2 |
| DCM::Version | 4.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-124 | Definitie van het concept Toelichting van de bouwsteen OverdrachtVaccinatie aanpassen. |
| ZIB-277 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet Voorschrijfcode (PRK) van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard Voorschrijfproducten (PRK). |
| ZIB-278 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet KNMP artikelnummer = ATKODE van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard Artikelen (Bestand 004). KNMP artikelnummer = ATKODE in tagged value notes geplaatst. |
| ZIB-279 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet Handelsproductcode (HPK) van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard HPK (Handels Product Kode). |
| ZIB-280 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet GTIN International Article Number van concept ProductCode aangepast naar tagged value DCM::CodeSystem GTIN (Global Trade Item Number). OID 2.16.528.1.1002 aangepast in 1.3.160. |
| ZIB-281 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet ATC (Anatomic Therapeutic Classification) van concept ProductCode aangepast naar tagged value DCM::CodeSystem ATC (Anatomic Therapeutic Classification). |
| ZIB-282 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet Generieke productcode (GPK) van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard GPK (Generieke Product Kode). |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 3.0 (01-05-2016)
| ZIB-453 | Wijziging naamgeving ZIB's en logo's door andere opzet van beheer |
Publicatieversie 3.1 (04-09-2017)
| ZIB-564 | Aanpassing/harmonisatie Engelse conceptnamen |
Publicatieversie 3.2 (01-10-2018)
| ZIB-664 | Bij Vaccinatie is alleen VaccinatieDatum verplicht? |
Publicatieversie 4.0 (06-07-2019)
| ZIB-821 | OverdrachtGeplandeZorgactiviteit laten vervallen en onderliggende zibs aanpassen |
Concept
Immunization can be defined as “Generating natural immunity against pathogens by means of vaccination (active immunization) or by administering immunoglobulins (passive immunization)”.
Only the (administered and planned) vaccinations are included in this information model. Administering immunoglobulins is part of the medication overview. Vaccinations have lifelong relevance.
Most vaccinations are carried out in the Netherlands as part of the RVP (Rijksvaccinatieprogramma, National Immunisation Program). RVP information is especially important for children.
Vaccinations are also relevant for adult patients such as transplant patients, dialysis patients and patients with a post-splenectomy status. In addition, there are specific indications for the vaccination of risk groups, such as travelers, professionals who come into contact with blood or patients with wounds, weakened immune systems or heightened risk.
Purpose
Documenting vaccinations that have already taken place in a patient or are planned is important for things such as the diagnostics of infectious diseases and the indication and planning of (re)vaccinations.
Evidence Base
Starting with version 4.0, the information model not only describes historical vaccinations, but also planned ones. Clinical reminders are also modeled with this information model. As a result, the concept PreferedDateForRevaccination has withdrawn. This date will now be the (vague) date of a planned vaccination.
Vaccination documentation contains far fewer data elements than medication documentation. Medication concerns a running order process with many participants (patient, prescriber, pharmacy, administrator) and complex, very diverse details.
Vaccinations are only about what was or will be administered and when.
Information Model
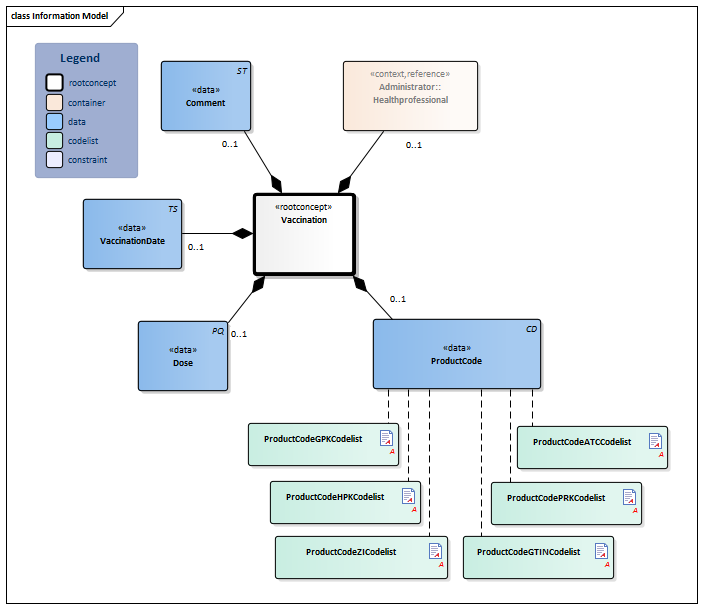
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||||||||||
| NL-CM:11.1.1 | Root concept of the Vaccination information model. This root concept contains all data elements of the Vaccination information model. | ||||||||||||||||||||||
| NL-CM:11.1.2 | 0..1 | The product code of the vaccine administered.
There are several possible code systems for documenting the product code. If the vaccination data is registered based on an anamnesis, coding with the ATC code is preferred. In all cases it concerns those products that fall under ATC group J07 (vaccines). |
| ||||||||||||||||||||
| NL-CM:11.1.4 | 0..1 | The amount of product administered shown in milliliters. In most cases, the entire product is administered; in some cases, a described part of the product is administered. | |||||||||||||||||||||
| NL-CM:11.1.3 | 0..1 | Date (and if possible time) that the vaccine was or will be administered. In the case of a planned re-vaccination, a vague date (month, year) is allowed (Clinical reminder). If a planned vaccination cannot be administered before a certain date, the date stated (vague or not) must indicate this. | |||||||||||||||||||||
| NL-CM:11.1.6 | 0..1 | The healthcare provider and/or organization where or by whom the immunization was done or will be done. |
| ||||||||||||||||||||
| NL-CM:11.1.7 | 0..1 | Free text explanation.
Examples of commonly used explanations are: - "Vaccination according to the National Immunisation Program". - "Not according to the National Immunisation Program", followed by further explanation. - "Unknown" |
|
||||||||||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| ProductCode | Vaccinatie Datum | Toelichting | GewensteDatum Hervaccinatie | Toediener |
| DKTP/HIB vaccin | 05-03-1999 | Volgens Rijksvaccinatie programma. | ||
| ProductCode | Vaccinatie Datum | Toelichting | GewensteDatum Hervaccinatie | Toediener |
| OrganisatieNaam | ||||
| Hepatitis A vaccin | 03-06-2012 | Bezoek aan Guatemala. | 2014 | GG&GD Nijmegen |
References
1. G-standaard [Online] Beschikbaar op: http://www.z-index.nl/ [Geraadpleegd: 23 februari 2015].
2. Rijksvaccinatieprogramma [Online] Beschikbaar op: http://www.rivm.nl/Onderwerpen/Onderwerpen/R/Rijksvaccinatieprogramma [Geraadpleegd: 23 februari 2015].
Valuesets
ProductCodeATCCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.4 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Anatomic Therapeutic Classification (ATC) | 2.16.840.1.113883.6.73 |
ProductCodeGPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.3 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Generieke Product Kode (GPK) | 2.16.840.1.113883.2.4.4.1 |
ProductCodeGTINCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.5 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Global Trade Item Number (GTIN) | 1.3.160 |
ProductCodeHPKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.2 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
ProductCodePRKCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.1 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Voorschrijfproducten (PRK) | 2.16.840.1.113883.2.4.4.10 |
ProductCodeZICodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.6 | Binding: Extensible |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | 2.16.840.1.113883.2.4.4.8 |
This information model in other releases
- Release 2015, (Version 1.2)
- Release 2016, (Version 3.0)
- Release 2017, (Version 3.1)
- Prerelease 2018-2, (Version 3.2)
- Release 2020, (Version 4.0)
- Prerelease 2021-2, (Version 4.1)
- Prerelease 2022-1, (Version 5.0)
- Prerelease 2023-1, (Version 6.0)
- Prerelease 2024-1, (Versie 6.0)
- Release 2024, (Version 6.0.1)
Information model references
This information model refers to
This information model is used in
- --
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2019-1
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms version: 20190131 [R] (January 2019 Release)
- LOINC version 2.64
Conditions for use are located on the mainpage ![]()
This page is generated on 07/07/2019 10:31:36 with ZibExtraction v. 3.0.7127.3724