MedicationUse-v1.0.1(2015EN)
General information
Name: nl.nfu.MedicationUse ![]()
Version: 1.0.1
HCIM Status:Final
Release: 2015
Release status: Published
Release date: 1-4-2015
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 19-12-2013 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | NFU |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.2 |
| DCM::KeywordList | Medicatie, Feitelijk Gebruik, Gebruik |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.nfu.MedicatieGebruik |
| DCM::PublicationDate | 1-4-2015 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 22-5-2015 |
| DCM::Superseeds | nl.nfu.OverdrachtMedicatie-v1.1 (2.16.840.1.113883.2.4.3.11.60.40.3.9.1) |
| DCM::Version | 1.0.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (01-04-2015)
| ZIB-56 | RFC Bouwsteen Medicatie |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Publicatieversie 1.0.1 (22-05-2015)
| ZIB-381 | Onterecht een spatie achter "OID :" in tagged value notes van tagged value DCM::ValueSet MedicatieGebruikRedenVanStoppenCodelijst van concept MedicatieGebruikRedenVanStoppen. |
Concept
MedicationUse describes taking or administering the medication, often in relation to a prescription, but also on the person’s own initiative. This describes the pattern of medication use, as reported by the patient themselves, a caregiver or healthcare provider. Documenting medication use provides insight into the use of prescribed medication as well as the use of medication at home.
Purpose
Recording medication information is a very important part of continuity in healthcare. It concerns the core of patient safety. Healthcare professionals in the collaborative branch must always have access to an up-to-date medication overview. Applying the building block will usually involve:
- Recording the patient’s intake of self-medication or ‘drugs’.
- Recording the medication used during a patient’s stay at the hospital.
- Medication verification: recording the active medication profile.
Information Model
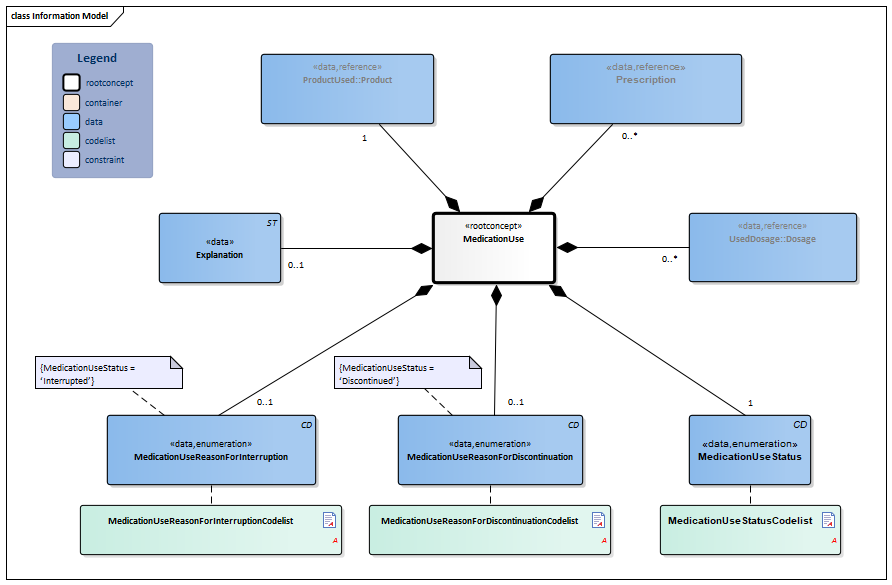
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||
| NL-CM:9.2.1 | Root concept of the MedicationUse building block. This root concept contains all data elements of the MedicationUse building block. | ||||||||||||
| NL-CM:9.2.2 | 1 | The product used. This is usually medication. Food, blood products, aids and bandages do not strictly fall under the category of medication, but can be recorded as well.
In principle, this will be the prescribed product, but the product used may differ from the prescribed product. |
| ||||||||||
| NL-CM:9.2.3 | 0..* | The agreement or order for the use of medication. |
| ||||||||||
| NL-CM:9.2.4 | 0..* | When taking stock of medication use, the dosage describes the amount and the pattern of use as reported by the patient or a healthcare provider.
The used dosage is the reported dose used by the patient. The used dosage may differ in terms of the administering schedule of the prescribed dosage in the event that the patient makes different decisions on their use of the product and reports as such. |
| ||||||||||
| NL-CM:9.2.5 | 1 | The status or status code is important in indicating the use schedule.
This attribute indicates whether the prescription is actively used, temporarily interrupted, or by now discontinued. Interrupting (home) use often occurs in the event of admittance to a healthcare facility, prior to a procedure and in response to monitoring (mirroring provisions, effect measurements, etc.). When documenting this, the following interpretations are used:
|
| ||||||||||
| NL-CM:9.2.6 | 0..1 | Reason why the use of a certain medicine was discontinued. |
| ||||||||||
| NL-CM:9.2.7 | 0..1 | Reason why the use of a certain medicine was interrupted. Here, you can choose to enter text or one of the codes. |
| ||||||||||
| NL-CM:9.2.8 | 0..1 | Comments on the medication use. | |||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| GebruiksProduct | GebruiksDosering | MedicatieGebruikStatus | Voorschrift | |||
| ProductNaam | StartDatum | EindDatum | Keerdosis | Toedieningsschema | ToedieningsWeg | Reden van Voorschrijven | |
| Probleem | ||||||
| Paracetamol tablet 500 mg | 05-2012 | Zo nodig 500mg (=1st), max. 4x/dag | Oraal | Actief | Hoofdpijn | |
| GebruiksProduct | GebruiksDosering | MedicatieGebruikStatus | Voorschrift | |||
| ProductNaam | StartDatum | EindDatum | Keerdosis | Toedieningsschema | ToedieningsWeg | Reden van Voorschrijven | |
| Probleem | ||||||
| Pantoprazol injpdr 40mg fl | 11-09-2012 17:21 | 1x/dag(8u) 40mg (=1st) | iv | Actief | Ulcusprofylaxe | |
| GebruiksProduct | GebruiksDosering | MedicatieGebruikStatus | Voorschrift | |||
| ProductNaam | StartDatum | EindDatum | Keerdosis | Toedieningsschema | ToedieningsWeg | Reden van Voorschrijven | |
| Probleem | ||||||
| Dalteparine 2500 injvlst 12.500 ie/ml wwsp 0,2ml | 19-09-2012 | 1x/dag(18u) 2500ie(=0,2ml) | Subcutaan | Actief | Thrombose- profylaxe | |
References
1. GROOT, E. (2011) Dataset medicatieproces 2011. [Online] Den Haag: Nictiz. Beschikbaar op: http://www.nictiz.nl/module/360/590/Dataset_Medicatieproces_2011.xlsx [Geraadpleegd: 23 juli 2014].
2. HL7v3-implementatiehandleiding medicatieproces versie 6.1.0.0. [Online] Den Haag: Nictiz. Beschikbaar op: http://www.nictiz.nl/uploaded/FILES/html_cabinet/live/Zorgtoepassing/Medicatieproces/AORTA_Mp_IH_Medicatieproces_HL7.htm [Geraadpleegd: 23 juli 2014].
3. Dossier Medicatieoverzicht. [Online] Beschikbaar op: Oria.nl. [Geraadpleegd: 23 juli 2014].
4. G-standaard documentatie. [Online] Beschikbaar op: http://www.z-index.nl/ [Geraadpleegd: 23 juli 2014].
Valuesets
MedicationUseReasonForDiscontinuationCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.2.2 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Intolerance | SINTOL | ActReason | 2.16.840.1.113883.5.8 | Bijwerking, allergie of intolerantie |
| Condition alert | COND | ActCode | 2.16.840.1.113883.5.4 | Contra-indicatie |
| Drug interacts with another drug | SDDI | ActReason | 2.16.840.1.113883.5.8 | Interactie met ander medicament |
| Dose change | DOSECHG | ActReason | 2.16.840.1.113883.5.8 | Dosiswijziging |
| No longer required for treatment | NOREQ | ActReason | 2.16.840.1.113883.5.8 | Niet langer vereist voor de behandeling |
| Ineffective | INEFFECT | ActReason | 2.16.840.1.113883.5.8 | Niet effectief |
| Formulary policy | FP | ActReason | 2.16.840.1.113883.5.8 | Ander voorschrijfbeleid |
| Product discontinued | DISCONT | ActReason | 2.16.840.1.113883.5.8 | Product niet meer leverbaar |
| Not covered | NOTCOVER | ActReason | 2.16.840.1.113883.5.8 | Product wordt niet vergoed |
| Patient refuse | PREFUS | ActReason | 2.16.840.1.113883.5.8 | Patiënt heeft geweigerd |
MedicationUseReasonForInterruptionCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.2.3 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Drug level too high | DRUGHIGH | ActReason | 2.16.840.1.113883.5.8 | Te hoge geneesmiddel spiegel |
| Lab interference issues | LABINT | ActReason | 2.16.840.1.113883.5.8 | Interferentie met gepland labonderzoek |
| Patient is pregnant/breast feeding | PREG | ActReason | 2.16.840.1.113883.5.8 | Patiënt is zwangerschap of geeft borstvoeding |
| Patient not-available | NON-AVAIL | ActReason | 2.16.840.1.113883.5.8 | Patiënt is niet beschikbaar |
| Response to monitoring | MONIT | ActReason | 2.16.840.1.113883.5.8 | Reactie op monitoring |
| Drug interacts with another drug | SDDI | ActReason | 2.16.840.1.113883.5.8 | Interactie met ander medicament |
| Duplicate therapy | SDUPTHER | ActReason | 2.16.840.1.113883.5.8 | Een andere therapie maakt het gebruik tijdelijk overbodig |
| Patient scheduled for surgery | SURG | ActReason | 2.16.840.1.113883.5.8 | Patient is ingepland voor een ingreep |
| Waiting for old drug to wash out | WASHOUT | ActReason | 2.16.840.1.113883.5.8 | Tijdelijk onderbreken tot ander geneesmiddel geen werking meer uitoefent |
MedicationUseStatusCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.2.1 |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Active | active | ActStatus | 2.16.840.1.113883.5.14 | Actief |
| Suspended | suspended | ActStatus | 2.16.840.1.113883.5.14 | Onderbroken |
| Aborted | aborted | ActStatus | 2.16.840.1.113883.5.14 | Afgebroken |
| Completed | completed | ActStatus | 2.16.840.1.113883.5.14 | Voltooid |
| Cancelled | cancelled | ActStatus | 2.16.840.1.113883.5.14 | Niet gestart |
This information model in other releases
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Registratie aan de bron publication 2015 including errata dd. 16-07-2015
Conditions for use are located on the mainpage ![]()
This page is generated on 24/01/2018 17:12:37