Vaccination-v1.2(2015EN): verschil tussen versies
Nieuwe pagina aangemaakt met '==General information== Name: '''nl.nfu.Vaccination''' link=Vaccinatie-v1.2(2015NL)<BR> Version: '''1.2''' <br> HCIM Status:Final<br> Publica...' |
Geen bewerkingssamenvatting |
||
| Regel 3: | Regel 3: | ||
Version: '''1.2''' <br> | Version: '''1.2''' <br> | ||
HCIM Status:Final<br> | HCIM Status:Final<br> | ||
Release: '''2015''' <br> | |||
Release status: Published<br> | Release status: Published<br> | ||
Release date: 1-4-2015 | |||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | ||
| Regel 143: | Regel 143: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
<imagemap> Bestand:Vaccination-v1.2Model(EN).png | center | |||
rect 566 410 717 453 [[#ProductCodeATCCodelist]] | |||
rect 540 466 691 509 [[#ProductCodePRKCodelist]] | |||
rect 512 520 663 563 [[#ProductCodeGTINCodelist]] | |||
rect 319 465 470 508 [[#ProductCodeHPKCodelist]] | |||
rect 324 520 497 563 [[#ProductCodeZICodelist]] | |||
rect 474 183 631 253 [[#9911]] | |||
rect 210 45 300 115 [[#9913]] | |||
rect 187 305 277 375 [[#9909]] | |||
rect 422 303 590 373 [[#9908]] | |||
rect 392 45 527 115 [[HealthProfessional-v1.2.1(2015EN)]] | |||
rect 131 183 230 253 [[#9910]] | |||
rect 303 178 403 258 [[#9907]] | |||
rect 297 407 448 450 [[#ProductCodeGPKCodelist]] | |||
desc none | |||
</imagemap> | |||
<BR> | <BR> | ||
{| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | {| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | ||
| Regel 149: | Regel 164: | ||
|style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | |style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | ||
|-style="vertical-align:top; background-color: #E3E3E3; " | |-style="vertical-align:top; background-color: #E3E3E3; " | ||
|style = "text-align:center" |[[Bestand: block.png| 20px]] | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | ||
||NL-CM:11.1.1 | ||NL-CM:11.1.1 | ||
|colspan ="6" style ="padding-left: 0px"|<span Title="NL: Vaccinatie">[[Bestand: arrowdown.png | 10px]]Vaccination</span> | |colspan ="6" style ="padding-left: 0px"|<span Id=9907 Title="NL: Vaccinatie">[[Bestand: arrowdown.png | 10px | link=]]Vaccination</span> | ||
| | | | ||
|Root concept of the Vaccination building block. This root concept contains all data elements of the Vaccination building block. | |Root concept of the Vaccination building block. This root concept contains all data elements of the Vaccination building block. | ||
| Regel 157: | Regel 172: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:11.1.2 | ||NL-CM:11.1.2 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ProductCode">[[Bestand: arrowright.png | 10px]]ProductCode</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9908 Title="NL: ProductCode">[[Bestand: arrowright.png | 10px | link=]]ProductCode</span> | ||
|0..1 | |0..1 | ||
|The product code of the vaccine administered. | |The product code of the vaccine administered. | ||
| Regel 170: | Regel 185: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeATCCodelist|ProductCodeATCCodelist]] | |[[Bestand: List2.png | link=#ProductCodeATCCodelist]]||[[#ProductCodeATCCodelist|ProductCodeATCCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeGPKCodelist|ProductCodeGPKCodelist]] | |[[Bestand: List2.png | link=#ProductCodeGPKCodelist]]||[[#ProductCodeGPKCodelist|ProductCodeGPKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeGTINCodelist|ProductCodeGTINCodelist]] | |[[Bestand: List2.png | link=#ProductCodeGTINCodelist]]||[[#ProductCodeGTINCodelist|ProductCodeGTINCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeHPKCodelist|ProductCodeHPKCodelist]] | |[[Bestand: List2.png | link=#ProductCodeHPKCodelist]]||[[#ProductCodeHPKCodelist|ProductCodeHPKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodePRKCodelist|ProductCodePRKCodelist]] | |[[Bestand: List2.png | link=#ProductCodePRKCodelist]]||[[#ProductCodePRKCodelist|ProductCodePRKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeZICodelist|ProductCodeZICodelist]] | |[[Bestand: List2.png | link=#ProductCodeZICodelist]]||[[#ProductCodeZICodelist|ProductCodeZICodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: PQ.png| 16px]] | |style = "text-align:center" |[[Bestand: PQ.png| 16px | link=]] | ||
||NL-CM:11.1.4 | ||NL-CM:11.1.4 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Dosis">[[Bestand: arrowright.png | 10px]]Dose</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9909 Title="NL: Dosis">[[Bestand: arrowright.png | 10px | link=]]Dose</span> | ||
|0..1 | |0..1 | ||
|The amount of product administered shown in milliliters. In most cases, the entire product is administered; in some cases, a described part of the product is administered. | |The amount of product administered shown in milliliters. In most cases, the entire product is administered; in some cases, a described part of the product is administered. | ||
| Regel 192: | Regel 207: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:11.1.3 | ||NL-CM:11.1.3 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: VaccinatieDatum">[[Bestand: arrowright.png | 10px]]VaccinationDate</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9910 Title="NL: VaccinatieDatum">[[Bestand: arrowright.png | 10px | link=]]VaccinationDate</span> | ||
|1 | |1 | ||
|Date (and if possible time) that the vaccine was administered. | |Date (and if possible time) that the vaccine was administered. | ||
| Regel 201: | Regel 216: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: TS.png| 16px]] | |style = "text-align:center" |[[Bestand: TS.png| 16px | link=]] | ||
||NL-CM:11.1.5 | ||NL-CM:11.1.5 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: GewensteDatumHervaccinatie">[[Bestand: arrowright.png | 10px]]DesiredDateForRevaccination</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9911 Title="NL: GewensteDatumHervaccinatie">[[Bestand: arrowright.png | 10px | link=]]DesiredDateForRevaccination</span> | ||
|0..1 | |0..1 | ||
|Date on which this vaccination will have to be repeated according to the author’s information. | |Date on which this vaccination will have to be repeated according to the author’s information. | ||
| Regel 210: | Regel 225: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: Verwijzing.png| 20px]] | |style = "text-align:center" |[[Bestand: Verwijzing.png| 20px | link=]] | ||
||NL-CM:11.1.6 | ||NL-CM:11.1.6 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Toediener::Zorgverlener">[[Bestand: arrowright.png | 10px]]Administrator::HealthcareProvider</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9912 Title="NL: Toediener::Zorgverlener">[[Bestand: arrowright.png | 10px | link=]]Administrator::HealthcareProvider</span> | ||
|0..1 | |0..1 | ||
|The healthcare provider and/or organization where or by whom the immunization was done. | |The healthcare provider and/or organization where or by whom the immunization was done. | ||
| Regel 220: | Regel 235: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: block.png]]||[[HealthProfessional-v1.2.1(2015EN) |HealthProfessional]] | |[[Bestand: block.png | link=HealthProfessional-v1.2.1(2015EN)]]||[[HealthProfessional-v1.2.1(2015EN) |HealthProfessional]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:11.1.7 | ||NL-CM:11.1.7 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: Toelichting">[[Bestand: arrowright.png | 10px]]Explanation</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=9913 Title="NL: Toelichting">[[Bestand: arrowright.png | 10px | link=]]Explanation</span> | ||
|0..1 | |0..1 | ||
|Free text explanation. | |Free text explanation. | ||
| Regel 246: | Regel 261: | ||
{|class="wikitable" width="749px" style= "font-size: 9.5pt;" | {|class="wikitable" width="749px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #1F497D; width: 20%; color | | style="background-color: #1F497D; width: 20%; "|<font color=#FFFFFF><b>ProductCode</b></font> | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Vaccinatie</b></font><font color=#FFFFFF><b>Datum</b></font> | ||
| style="background-color: #1F497D; width: 26%; color | | style="background-color: #1F497D; width: 26%; "|<font color=#FFFFFF><b>Toelichting</b></font> | ||
| style="background-color: #1F497D; width: 20%; color | | style="background-color: #1F497D; width: 20%; "|<font color=#FFFFFF><b>Gewenste</b></font><font color=#FFFFFF><b>Datum</b></font><font color=#FFFFFF><b> </b></font><font color=#FFFFFF><b>H</b></font><font color=#FFFFFF><b>ervaccinatie</b></font> | ||
| style="background-color: #1F497D; width: 21%; color | | style="background-color: #1F497D; width: 21%; "|<font color=#FFFFFF><b>Toediener</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 20%; "| | | style="background-color: #548DD4; width: 20%; "| | ||
| Regel 270: | Regel 285: | ||
| style="width: 21%; "|<BR> | | style="width: 21%; "|<BR> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #1F497D; width: 20%; color | | style="background-color: #1F497D; width: 20%; "|<font color=#FFFFFF><b>ProductCode</b></font> | ||
| style="background-color: #1F497D; width: 13%; color | | style="background-color: #1F497D; width: 13%; "|<font color=#FFFFFF><b>Vaccinatie</b></font><font color=#FFFFFF><b>Datum</b></font> | ||
| style="background-color: #1F497D; width: 26%; color | | style="background-color: #1F497D; width: 26%; "|<font color=#FFFFFF><b>Toelichting</b></font> | ||
| style="background-color: #1F497D; width: 20%; color | | style="background-color: #1F497D; width: 20%; "|<font color=#FFFFFF><b>GewensteDatum Hervaccinatie</b></font> | ||
| style="background-color: #1F497D; width: 21%; color | | style="background-color: #1F497D; width: 21%; "|<font color=#FFFFFF><b>Toediener</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 20%; "| | | style="background-color: #548DD4; width: 20%; "| | ||
| Regel 280: | Regel 295: | ||
| style="background-color: #548DD4; width: 26%; "| | | style="background-color: #548DD4; width: 26%; "| | ||
| style="background-color: #548DD4; width: 20%; "| | | style="background-color: #548DD4; width: 20%; "| | ||
| style="background-color: #548DD4; width: 21%; color | | style="background-color: #548DD4; width: 21%; "|<font color=#FFFFFF><b>OrganisatieNaam</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="width: 20%; "|Hepatitis A vaccin | | style="width: 20%; "|Hepatitis A vaccin | ||
| Regel 386: | Regel 401: | ||
==This information model in other releases== | |||
<ul> | |||
<li>[[Vaccination-v3.0(2016EN) | Release 2016, (Version 3.0)]]</li> | |||
<li>[[Vaccination-v3.1(2017EN) | Release 2017, (Version 3.1)]]</li> | |||
</ul> | |||
==More on this information model== | ==More on this information model== | ||
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | |||
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications: | |||
<ul> | |||
<li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment [http://decor.nictiz.nl/art-decor/decor-scenarios--zib2015bbr-?id=2.16.840.1.113883.2.4.3.11.60.5.4.2.11.1&effectiveDate=2015-04-01T00:00:00&language=en-US&scenariotree=false [[File:artdecor.jpg|16px|link=]]]</li> | |||
<li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR [https://simplifier.net/NictizSTU3/~resources?text=zib&category=StructureDefinition [[File:fhir.png|link=]]]</li> | |||
</ul> | |||
This information model is also available as [[Media:nl.nfu.Vaccination-v1.2(2015EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.nfu.Vaccination-v1.2(2015EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | This information model is also available as [[Media:nl.nfu.Vaccination-v1.2(2015EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.nfu.Vaccination-v1.2(2015EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | ||
==About this information== | ==About this information== | ||
The information in this wikipage is based on ''Registratie aan de bron'' publication 2015 including errata dd. 16-07-2015 <BR> | The information in this wikipage is based on ''Registratie aan de bron'' publication 2015 including errata dd. 16-07-2015 <BR> | ||
Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | ||
This page is generated on | This page is generated on 24/01/2018 17:14:15 <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2015(EN)]] [[HCIM_Release_2015(EN) |Back to HCIM list ]]</div> | ||
Versie van 25 jan 2018 00:20
General information
Name: nl.nfu.Vaccination ![]()
Version: 1.2
HCIM Status:Final
Release: 2015
Release status: Published
Release date: 1-4-2015
Metadata
| DCM::CoderList | Kerngroep Registratie aan de Bron |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::CreationDate | 5-10-2012 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | NFU |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.11.1 |
| DCM::KeywordList | vaccinatie, inenting |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Kerngroep Registratie aan de Bron |
| DCM::Name | nl.nfu.Vaccinatie |
| DCM::PublicationDate | 1-4-2015 |
| DCM::PublicationStatus | Published |
| DCM::ReviewerList | Projectgroep Generieke Overdrachtsgegevens & Kerngroep Registratie aan de Bron |
| DCM::RevisionDate | 1-4-2015 |
| DCM::Superseeds | |
| DCM::Version | 1.2 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (15-02-2013)
Publicatieversie 1.1 (01-07-2013)
Publicatieversie 1.2 (01-04-2015)
| ZIB-124 | Definitie van het concept Toelichting van de bouwsteen OverdrachtVaccinatie aanpassen. |
| ZIB-277 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet Voorschrijfcode (PRK) van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard Voorschrijfproducten (PRK). |
| ZIB-278 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet KNMP artikelnummer = ATKODE van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard Artikelen (Bestand 004). KNMP artikelnummer = ATKODE in tagged value notes geplaatst. |
| ZIB-279 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet Handelsproductcode (HPK) van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard HPK (Handels Product Kode). |
| ZIB-280 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet GTIN International Article Number van concept ProductCode aangepast naar tagged value DCM::CodeSystem GTIN (Global Trade Item Number). OID 2.16.528.1.1002 aangepast in 1.3.160. |
| ZIB-281 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet ATC (Anatomic Therapeutic Classification) van concept ProductCode aangepast naar tagged value DCM::CodeSystem ATC (Anatomic Therapeutic Classification). |
| ZIB-282 | In de klinische bouwsteen OverdrachtVaccinatie DCM::ValueSet Generieke productcode (GPK) van concept ProductCode aangepast naar tagged value DCM::CodeSystem G-Standaard GPK (Generieke Product Kode). |
| ZIB-308 | Prefix Overdracht weggehaald bij de generieke bouwstenen |
| ZIB-353 | Tagged values DCM::CodeSystem aanpassen naar DCM::ValueSet incl. gekoppelde codelijst. |
Incl. algemene wijzigingsverzoeken:
| ZIB-94 | Aanpassen tekst van Disclaimer, Terms of Use & Copyrights |
| ZIB-154 | Consequenties opsplitsing Medicatie bouwstenen voor overige bouwstenen. |
| ZIB-200 | Naamgeving SNOMED CT in tagged values klinische bouwstenen gelijk getrokken. |
| ZIB-201 | Naamgeving OID: in tagged value notes van klinische bouwstenen gelijk getrokken. |
| ZIB-309 | EOI aangepast |
| ZIB-324 | Codelijsten Name en Description beginnen met een Hoofdletter |
| ZIB-326 | Tekstuele aanpassingen conform de kwaliteitsreview kerngroep 2015 |
Concept
Immunization can be defined as “Generating natural immunity against pathogens by means of vaccination (active immunization) or by administering immunoglobulins (passive immunization)”.
Only vaccinations are included in this building block. Administering immunoglobulins is part of the medication overview. Vaccinations have lifelong relevance.
Most vaccinations are carried out in the Netherlands as part of the RVP (Rijksvaccinatieprogramma, National Immunisation Program). RVP information is especially important for children.
Vaccinations are also relevant for adult patients such as transplant patients, dialysis patients and patients with a post-splenectomy status. In addition, there are specific indications for the vaccination of risk groups, such as travelers, professionals who come into contact with blood or patients with wounds, weakened immune systems or heightened risk.
Purpose
Documenting vaccinations that have already taken place in a patient is important for things such as the diagnostics of infectious diseases and the indication and planning of (re)vaccinations.
Evidence Base
Vaccination documentation contains far fewer data elements than medication documentation. Medication concerns a running order process with many participants (patient, prescriber, pharmacy, administrator) and complex, very diverse details.
Vaccinations are only about what was administered and when.
Information Model
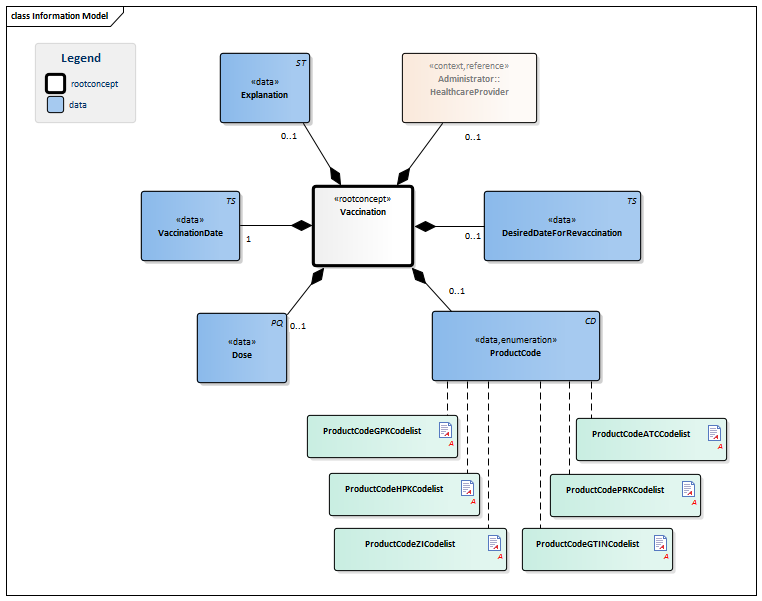
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||||||||||
| NL-CM:11.1.1 | Root concept of the Vaccination building block. This root concept contains all data elements of the Vaccination building block. | ||||||||||||||||||||||
| NL-CM:11.1.2 | 0..1 | The product code of the vaccine administered.
There are several possible code systems for documenting the product code. If the vaccination data is registered based on an anamnesis, coding with the ATC code is preferred. In all cases it concerns those products that fall under ATC group J07 (vaccines). |
| ||||||||||||||||||||
| NL-CM:11.1.4 | 0..1 | The amount of product administered shown in milliliters. In most cases, the entire product is administered; in some cases, a described part of the product is administered. | |||||||||||||||||||||
| NL-CM:11.1.3 | 1 | Date (and if possible time) that the vaccine was administered. | |||||||||||||||||||||
| NL-CM:11.1.5 | 0..1 | Date on which this vaccination will have to be repeated according to the author’s information. | |||||||||||||||||||||
| NL-CM:11.1.6 | 0..1 | The healthcare provider and/or organization where or by whom the immunization was done. |
| ||||||||||||||||||||
| NL-CM:11.1.7 | 0..1 | Free text explanation.
Examples of commonly used explanations are: - "Vaccination according to the National Immunisation Program". - "Not according to the National Immunisation Program", followed by further explanation. - "Unknown" |
|||||||||||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| ProductCode | VaccinatieDatum | Toelichting | GewensteDatum Hervaccinatie | Toediener |
| DKTP/HIB vaccin | 05-03-1999 | Volgens Rijksvaccinatie programma. | ||
| ProductCode | VaccinatieDatum | Toelichting | GewensteDatum Hervaccinatie | Toediener |
| OrganisatieNaam | ||||
| Hepatitis A vaccin | 03-06-2012 | Bezoek aan Guatemala. | 2014 | GG&GD Nijmegen |
References
1. G-standaard [Online] Beschikbaar op: http://www.z-index.nl/ [Geraadpleegd: 23 februari 2015].
2. Rijksvaccinatieprogramma [Online] Beschikbaar op: http://www.rivm.nl/Onderwerpen/Onderwerpen/R/Rijksvaccinatieprogramma [Geraadpleegd: 23 februari 2015].
Valuesets
ProductCodeATCCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.4 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Anatomic Therapeutic Classification (ATC) | 2.16.840.1.113883.6.73 |
ProductCodeGPKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.3 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Generieke Product Kode (GPK) | 2.16.840.1.113883.2.4.4.1 |
ProductCodeGTINCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.5 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Global Trade Item Number (GTIN) | 1.3.160 |
ProductCodeHPKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.2 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
ProductCodePRKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.1 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Voorschrijfproducten (PRK) | 2.16.840.1.113883.2.4.4.10 |
ProductCodeZICodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.11.1.6 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | 2.16.840.1.113883.2.4.4.8 |
This information model in other releases
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Registratie aan de bron publication 2015 including errata dd. 16-07-2015
Conditions for use are located on the mainpage ![]()
This page is generated on 24/01/2018 17:14:15