Product-v1.0(2017EN): verschil tussen versies
Geen bewerkingssamenvatting |
Geen bewerkingssamenvatting |
||
| (Een tussenliggende versie door dezelfde gebruiker niet weergegeven) | |||
| Regel 3: | Regel 3: | ||
Version: '''1.0''' <br> | Version: '''1.0''' <br> | ||
HCIM Status:Final<br> | HCIM Status:Final<br> | ||
Release: '''2017''' <br> | |||
Release status: Prepublished<br> | Release status: Prepublished<br> | ||
Release date: 04-09-2017 | |||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | ||
| Regel 85: | Regel 85: | ||
==Information Model== | ==Information Model== | ||
<BR> | <BR> | ||
<imagemap> Bestand:Product-v1.0Model(EN).png | center | |||
rect 418 647 529 727 [[#13249]] | |||
rect 559 647 678 727 [[#13260]] | |||
rect 108 820 280 870 [[#IngredientCodeGTINCodelist]] | |||
rect 723 373 899 423 [[#ProductCodeATCCodelist]] | |||
rect 108 752 280 802 [[#IngredientCodeSSKCodelist]] | |||
rect 108 626 280 676 [[#IngredientCodeATCCodelist]] | |||
rect 108 191 285 241 [[#Pharmaceutical formCodelist]] | |||
rect 108 689 280 739 [[#IngredientCodeSNKCodelist]] | |||
rect 108 563 280 613 [[#IngredientCodeZICodelist]] | |||
rect 108 500 280 550 [[#IngredientCodePRKCodelist]] | |||
rect 108 438 280 488 [[#IngredientCodeHPKCodelist]] | |||
rect 105 377 277 427 [[#IngredientCodeGPKCodelist]] | |||
rect 723 303 899 353 [[#ProductCodeZICodelist]] | |||
rect 723 236 899 286 [[#ProductCodeGTINCodelist]] | |||
rect 722 169 898 219 [[#ProductCodePRKCodelist]] | |||
rect 723 102 899 152 [[#ProductCodeHPKCodelist]] | |||
rect 723 35 899 85 [[#ProductCodeGPKCodelist]] | |||
rect 406 276 510 346 [[#13251]] | |||
rect 338 533 428 603 [[#13268]] | |||
rect 460 533 550 603 [[#13273]] | |||
rect 408 408 498 478 [[#13259]] | |||
rect 239 277 352 347 [[#13272]] | |||
rect 569 357 659 427 [[#13253]] | |||
rect 568 162 658 232 [[#13248]] | |||
rect 568 255 658 325 [[#13252]] | |||
rect 411 143 501 213 [[#13255]] | |||
desc none | |||
</imagemap> | |||
<BR> | <BR> | ||
{| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | {| border= "1" width="1500px" style = "font-size: 9.5pt; border: solid 1px silver; border-collapse:collapse;" cellpadding = "3px" cellspacing ="0px" | ||
| Regel 91: | Regel 119: | ||
|style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | |style="width:30px;"|Type||style="width:100px;"|Id||colspan="6 " style="width:140px;"|Concept||Card.||style="width: 600px;"|Definition||style="width:200px;"|DefinitionCode||style="width:200px;"|Reference | ||
|-style="vertical-align:top; background-color: #E3E3E3; " | |-style="vertical-align:top; background-color: #E3E3E3; " | ||
|style = "text-align:center" |[[Bestand: block.png| 20px]] | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | ||
||NL-CM:9.7.19926 | ||NL-CM:9.7.19926 | ||
|colspan ="6" style ="padding-left: 0px"|<span Title="NL: Product">[[Bestand: arrowdown.png | 10px]]Product</span> | |colspan ="6" style ="padding-left: 0px"|<span Id=13255 Title="NL: Product">[[Bestand: arrowdown.png | 10px | link=]]Product</span> | ||
| | | | ||
|Root concept of the Product partial information model. This root concept contains all data elements of the Product partial information model. | |Root concept of the Product partial information model. This root concept contains all data elements of the Product partial information model. | ||
| Regel 112: | Regel 140: | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:9.7.19928 | ||NL-CM:9.7.19928 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ProductSpecificatie">[[Bestand: arrowdown.png | 10px]]ProductSpecifications</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=13251 Title="NL: ProductSpecificatie">[[Bestand: arrowdown.png | 10px | link=]]ProductSpecifications</span> | ||
|0..1 | |0..1 | ||
|Container of the ProductSpecifications concept. This container contains all data elements of the ProductSpecifications concept. | |Container of the ProductSpecifications concept. This container contains all data elements of the ProductSpecifications concept. | ||
| Regel 124: | Regel 152: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:9.7.19931 | ||NL-CM:9.7.19931 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: FarmaceutischeVorm">[[Bestand: arrowright.png | 10px]]PharmaceuticalForm</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=13272 Title="NL: FarmaceutischeVorm">[[Bestand: arrowright.png | 10px | link=]]PharmaceuticalForm</span> | ||
|0..1 | |0..1 | ||
|The pharmaceutical form indicates the form of the medication in accordance with the route of administration. Examples include: tablet, suppository, infusion liquid, ointment. If the product has a generic code in the G standard, the form will be known in the G standard. For products without a code (free text, preparation by the pharmacy), the means of administration can be entered. | |The pharmaceutical form indicates the form of the medication in accordance with the route of administration. Examples include: tablet, suppository, infusion liquid, ointment. If the product has a generic code in the G standard, the form will be known in the G standard. For products without a code (free text, preparation by the pharmacy), the means of administration can be entered. | ||
| Regel 135: | Regel 163: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#Pharmaceutical formCodelist|Pharmaceutical formCodelist]] | |[[Bestand: List2.png | link=#Pharmaceutical formCodelist]]||[[#Pharmaceutical formCodelist|Pharmaceutical formCodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:9.7.19927 | ||NL-CM:9.7.19927 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Title="NL: ProductCode">[[Bestand: arrowright.png | 10px]]MedicationCode</span> | |colspan ="5" style ="padding-left: 0px"|<span Id=13248 Title="NL: ProductCode">[[Bestand: arrowright.png | 10px | link=]]MedicationCode</span> | ||
|0..1 | |0..1 | ||
|Coding medication in the Netherlands is done on the basis of the G standard (issued by Z-index), which is filled under the direction of KNMP. | |Coding medication in the Netherlands is done on the basis of the G standard (issued by Z-index), which is filled under the direction of KNMP. | ||
| Regel 177: | Regel 205: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeATCCodelist|ProductCodeATCCodelist]] | |[[Bestand: List2.png | link=#ProductCodeATCCodelist]]||[[#ProductCodeATCCodelist|ProductCodeATCCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeGPKCodelist|ProductCodeGPKCodelist]] | |[[Bestand: List2.png | link=#ProductCodeGPKCodelist]]||[[#ProductCodeGPKCodelist|ProductCodeGPKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeGTINCodelist|ProductCodeGTINCodelist]] | |[[Bestand: List2.png | link=#ProductCodeGTINCodelist]]||[[#ProductCodeGTINCodelist|ProductCodeGTINCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeHPKCodelist|ProductCodeHPKCodelist]] | |[[Bestand: List2.png | link=#ProductCodeHPKCodelist]]||[[#ProductCodeHPKCodelist|ProductCodeHPKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodePRKCodelist|ProductCodePRKCodelist]] | |[[Bestand: List2.png | link=#ProductCodePRKCodelist]]||[[#ProductCodePRKCodelist|ProductCodePRKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#ProductCodeZICodelist|ProductCodeZICodelist]] | |[[Bestand: List2.png | link=#ProductCodeZICodelist]]||[[#ProductCodeZICodelist|ProductCodeZICodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:9.7.19929 | ||NL-CM:9.7.19929 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: ProductNaam">[[Bestand: arrowright.png | 10px]]Medication</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=13252 Title="NL: ProductNaam">[[Bestand: arrowright.png | 10px | link=]]Medication</span> | ||
|0..1 | |0..1 | ||
|There is no code for medication entered in free text. In these cases, enter the complete description. | |There is no code for medication entered in free text. In these cases, enter the complete description. | ||
| Regel 200: | Regel 228: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: ST.png| 16px]] | |style = "text-align:center" |[[Bestand: ST.png| 16px | link=]] | ||
||NL-CM:9.7.19784 | ||NL-CM:9.7.19784 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Omschrijving">[[Bestand: arrowright.png | 10px]]Description</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=13253 Title="NL: Omschrijving">[[Bestand: arrowright.png | 10px | link=]]Description</span> | ||
|0..1 | |0..1 | ||
|A textual description of the type of medication (including relevant properties of the composition and preparation method if possible), which is only used if no coded indication from the G Standard is available (magistral preparation). | |A textual description of the type of medication (including relevant properties of the composition and preparation method if possible), which is only used if no coded indication from the G Standard is available (magistral preparation). | ||
| Regel 210: | Regel 238: | ||
| | | | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:9.7.19932 | ||NL-CM:9.7.19932 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Title="NL: Ingredient">[[Bestand: arrowdown.png | 10px]]Ingredient</span> | |colspan ="4" style ="padding-left: 0px"|<span Id=13259 Title="NL: Ingredient">[[Bestand: arrowdown.png | 10px | link=]]Ingredient</span> | ||
|0..* | |0..* | ||
|Container of the Ingredient concept. This container contains all data elements of the Ingredient concept. | |Container of the Ingredient concept. This container contains all data elements of the Ingredient concept. | ||
| Regel 227: | Regel 255: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: CD.png| 16px]] | |style = "text-align:center" |[[Bestand: CD.png| 16px | link=]] | ||
||NL-CM:9.7.19934 | ||NL-CM:9.7.19934 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="3" style ="padding-left: 0px"|<span Title="NL: IngredientCode">[[Bestand: arrowright.png | 10px]]SubstanceCode</span> | |colspan ="3" style ="padding-left: 0px"|<span Id=13268 Title="NL: IngredientCode">[[Bestand: arrowright.png | 10px | link=]]SubstanceCode</span> | ||
|0..1 | |0..1 | ||
|Active substance or excipient. | |Active substance or excipient. | ||
| Regel 253: | Regel 281: | ||
{| | {| | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeATCCodelist|IngredientCodeATCCodelist]] | |[[Bestand: List2.png | link=#IngredientCodeATCCodelist]]||[[#IngredientCodeATCCodelist|IngredientCodeATCCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeGPKCodelist|IngredientCodeGPKCodelist]] | |[[Bestand: List2.png | link=#IngredientCodeGPKCodelist]]||[[#IngredientCodeGPKCodelist|IngredientCodeGPKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeGTINCodelist|IngredientCodeGTINCodelist]] | |[[Bestand: List2.png | link=#IngredientCodeGTINCodelist]]||[[#IngredientCodeGTINCodelist|IngredientCodeGTINCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeHPKCodelist|IngredientCodeHPKCodelist]] | |[[Bestand: List2.png | link=#IngredientCodeHPKCodelist]]||[[#IngredientCodeHPKCodelist|IngredientCodeHPKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodePRKCodelist|IngredientCodePRKCodelist]] | |[[Bestand: List2.png | link=#IngredientCodePRKCodelist]]||[[#IngredientCodePRKCodelist|IngredientCodePRKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeSNKCodelist|IngredientCodeSNKCodelist]] | |[[Bestand: List2.png | link=#IngredientCodeSNKCodelist]]||[[#IngredientCodeSNKCodelist|IngredientCodeSNKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeSSKCodelist|IngredientCodeSSKCodelist]] | |[[Bestand: List2.png | link=#IngredientCodeSSKCodelist]]||[[#IngredientCodeSSKCodelist|IngredientCodeSSKCodelist]] | ||
|- | |- | ||
|[[Bestand: List2.png]]||[[#IngredientCodeZICodelist|IngredientCodeZICodelist]] | |[[Bestand: List2.png | link=#IngredientCodeZICodelist]]||[[#IngredientCodeZICodelist|IngredientCodeZICodelist]] | ||
|} | |} | ||
|-style="vertical-align:top; background-color: #E8D7BE; " | |-style="vertical-align:top; background-color: #E8D7BE; " | ||
|style = "text-align:center" |[[Bestand: folder.png| 16px]] | |style = "text-align:center" |[[Bestand: folder.png| 16px | link=]] | ||
||NL-CM:9.7.19933 | ||NL-CM:9.7.19933 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="3" style ="padding-left: 0px"|<span Title="NL: Sterkte">[[Bestand: arrowdown.png | 10px]]Concentration</span> | |colspan ="3" style ="padding-left: 0px"|<span Id=13273 Title="NL: Sterkte">[[Bestand: arrowdown.png | 10px | link=]]Concentration</span> | ||
|0..1 | |0..1 | ||
|The relative amount of this ingredient in this product. | |The relative amount of this ingredient in this product. | ||
| Regel 284: | Regel 312: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: PQ.png| 16px]] | |style = "text-align:center" |[[Bestand: PQ.png| 16px | link=]] | ||
||NL-CM:9.7.22277 | ||NL-CM:9.7.22277 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
| Regel 290: | Regel 318: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="2" style ="padding-left: 0px"|<span Title="NL: IngredientHoeveelheid">[[Bestand: arrowright.png | 10px]]IngredientAmount</span> | |colspan ="2" style ="padding-left: 0px"|<span Id=13260 Title="NL: IngredientHoeveelheid">[[Bestand: arrowright.png | 10px | link=]]IngredientAmount</span> | ||
|0..1 | |0..1 | ||
|The amount of this ingredient. This is the numerator for the calculation of the concentration. The unit should be selected from the G-Standard (Table 902). | |The amount of this ingredient. This is the numerator for the calculation of the concentration. The unit should be selected from the G-Standard (Table 902). | ||
| Regel 296: | Regel 324: | ||
| | | | ||
|-style="vertical-align:top; background-color: transparent; " | |-style="vertical-align:top; background-color: transparent; " | ||
|style = "text-align:center" |[[Bestand: PQ.png| 16px]] | |style = "text-align:center" |[[Bestand: PQ.png| 16px | link=]] | ||
||NL-CM:9.7.22278 | ||NL-CM:9.7.22278 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
| Regel 302: | Regel 330: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="2" style ="padding-left: 0px"|<span Title="NL: ProductHoeveelheid">[[Bestand: arrowright.png | 10px]]ProductAmount</span> | |colspan ="2" style ="padding-left: 0px"|<span Id=13249 Title="NL: ProductHoeveelheid">[[Bestand: arrowright.png | 10px | link=]]ProductAmount</span> | ||
|0..1 | |0..1 | ||
|Amount of the product. This is the denominator for the calculation of the concentration. | |Amount of the product. This is the denominator for the calculation of the concentration. | ||
| Regel 316: | Regel 344: | ||
{|class="wikitable" width="286px" style= "font-size: 9.5pt;" | {|class="wikitable" width="286px" style= "font-size: 9.5pt;" | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #1F497D; width: 100%; color | | style="background-color: #1F497D; width: 100%; "|<font color=#FFFFFF><b>Afgesproken geneesmiddel</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #548DD4; width: 100%; color | | style="background-color: #548DD4; width: 100%; "|<font color=#FFFFFF><b>Product</b></font> | ||
|-style=vertical-align:top; | |-style=vertical-align:top; | ||
| style="background-color: #D9D9D9; width: 100%; "| | | style="background-color: #D9D9D9; width: 100%; "| | ||
| Regel 554: | Regel 582: | ||
==This information model in other releases== | |||
--- | |||
==More on this information model== | ==More on this information model== | ||
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | |||
This information model is also available as [[Media:nl.zorg.part.Product-v1.0( | Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications: | ||
<ul> | |||
<li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment [http://decor.nictiz.nl/art-decor/decor-scenarios--zib2017bbr-?id=2.16.840.1.113883.2.4.3.11.60.7.4.2.9.7&effectiveDate=2017-09-04T00:00:00&language=en-US&scenariotree=false [[File:artdecor.jpg|16px|link=]]]</li> | |||
<li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR [https://simplifier.net/NictizSTU3/~resources?text=zib&category=StructureDefinition [[File:fhir.png|link=]]]</li> | |||
</ul> | |||
This information model is also available as [[Media:nl.zorg.part.Product-v1.0(2017EN).pdf|pdf file]] [[File:PDF.png|link=]] or as [[Media:nl.zorg.part.Product-v1.0(2017EN).xlsx|spreadsheet]] [[File:xlsx.png|link=]] | |||
==About this information== | ==About this information== | ||
The information in this wikipage is based on Prerelease 2017 #1 <BR> | The information in this wikipage is based on Prerelease 2017 #1 <BR> | ||
Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | Conditions for use are located on the mainpage [[Bestand:list2.png|link=HCIM_Mainpage]]<BR> | ||
This page is generated on | This page is generated on 30/11/2017 13:54:00 <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release_2017(EN)]] [[HCIM_Release_2017(EN) |Back to HCIM list ]]</div> | ||
Huidige versie van 30 nov 2017 om 13:54
General information
Name: nl.zorg.part.Product ![]()
Version: 1.0
HCIM Status:Final
Release: 2017
Release status: Prepublished
Release date: 04-09-2017
Metadata
| DCM::CoderList | Projectgroep Medicatieproces |
| DCM::ContactInformation.Address | |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | |
| DCM::ContentAuthorList | Projectgroep Medicatieproces |
| DCM::CreationDate | 1-3-2017 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | PM |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.9.7 |
| DCM::KeywordList | Product |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Architectuurgroep Registratie aan de Bron |
| DCM::Name | nl.zorg.part.Product |
| DCM::PublicationDate | 04-09-2017 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | Projectgroep Medicatieproces & Architectuurgroep Registratie aan de Bron |
| DCM::RevisionDate | 04-09-2017 |
| DCM::Superseeds | |
| DCM::Version | 1.0 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (04-09-2017).
Concept
The prescribed substance is usually medication. However, medical aids and bandages can also be prescribed and supplied via the pharmacy. Food and blood products do not strictly belong in the medication category, but can be prescribed and supplied by a pharmacy as well.
A type of medication can be indicated with a single code. That code can be chosen from several possible coding systems (concretely: GPK, PRK, HPK or article numbers). Correct use of these codes in the software systems will sufficiently record the composition of the product used, making a complete product specification unnecessary.
In addition to a primary code, alternative codes from other coding systems can also be entered (so that the GPK can be sent along in the event that the patient was registered based on PRK, for example).
Entering multiple ingredients will enable you to display a compound product. If one of the composite parts is liquid, the dosage will be given in milliliters; otherwise it will be given in ‘units’.
In that case, the composition of the medication can be specified implicitly (with the use of a medication code) or explicitly, for example by listing the (active) ingredient(s) of the medication.
Magistral prescriptions can be entered as well. This can be done by means of the option listed above to enter coded ingredients and/or by entering the composition and preparation method as free text.
This is a partial information model
Purpose
The purpose of Product is to unambiguously describe the medication to be used.
Information Model
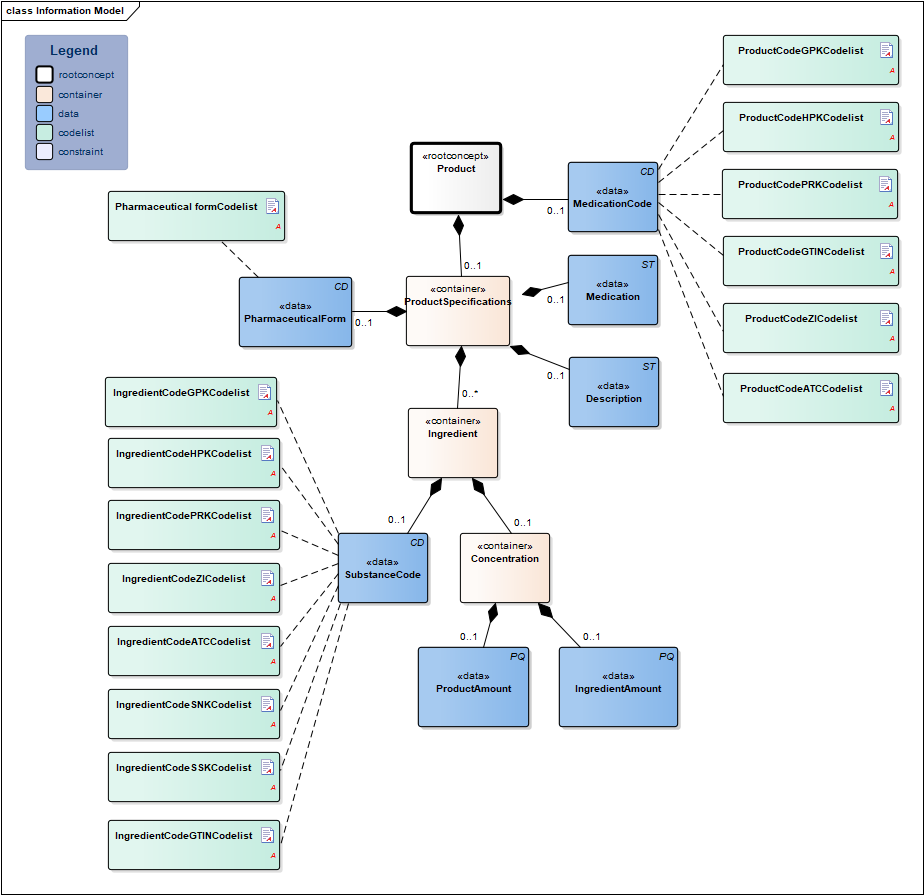
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||||||||||
| NL-CM:9.7.19926 | Root concept of the Product partial information model. This root concept contains all data elements of the Product partial information model.
The prescribed product is usually a medicine. However, medical aids and bandages can also be prescribed and supplied via the pharmacy. Strictly speaking, food and blood products do not belong in the medication category, but can be prescribed and supplied by a pharmacy as well. A type of medication can be indicated with a single code. That code can be chosen from several possible coding systems (concretely: GPK, PRK, HPK or article numbers). Correct use of these codes in the software systems will sufficiently record the composition of the product used, making a complete product specification unnecessary. In addition to a primary code, alternative codes from other coding systems can also be entered (so that the GPK can be sent along in the event that the patient was registered based on PRK, for example). Entering multiple ingredients will enable you to display a compound product. If one of the composite parts is liquid, the dosage will be given in milliliters; otherwise it will be given in ‘units’. In that case, the composition of the medication can be specified implicitly (with the use of a medication code) or explicitly, for example by listing the (active) substance(s) of the medication. Prescriptions to be prepared by the pharmacy can be entered as well. This can be done by means of the option listed above to enter coded ingredients and/or by entering the composition and preparation method as free text. |
||||||||||||||||||||||
| NL-CM:9.7.19928 | 0..1 | Container of the ProductSpecifications concept. This container contains all data elements of the ProductSpecifications concept.
Product specifications are required if the product code is not sufficient to ascertain the active substances and strength. |
|||||||||||||||||||||
| NL-CM:9.7.19931 | 0..1 | The pharmaceutical form indicates the form of the medication in accordance with the route of administration. Examples include: tablet, suppository, infusion liquid, ointment. If the product has a generic code in the G standard, the form will be known in the G standard. For products without a code (free text, preparation by the pharmacy), the means of administration can be entered. |
| ||||||||||||||||||||
| NL-CM:9.7.19927 | 0..1 | Coding medication in the Netherlands is done on the basis of the G standard (issued by Z-index), which is filled under the direction of KNMP.
The coded medication can be expressed as:
The prescription code (PRK) was developed and added to the older generic (GPK) and supplier-specific (HPK, ATKODE) coding to enable a generic product to be entered without listing a specific brand on the one hand, and to enable providing enough information to support the pharmacy supplying it on the other. The Substance Name Code (SNK) and the Substance Name Code, in combination with Route of Administration (SSK) are used to prescribe at a more generic level. The GTIN coding is used for the implementation of a barcode scanning standard and to be able to trace the origin of the product. The 90.000.000 number is used in accordance with national agreements. |
| ||||||||||||||||||||
| NL-CM:9.7.19929 | 0..1 | There is no code for medication entered in free text. In these cases, enter the complete description. | |||||||||||||||||||||
| NL-CM:9.7.19784 | 0..1 | A textual description of the type of medication (including relevant properties of the composition and preparation method if possible), which is only used if no coded indication from the G Standard is available (magistral preparation). | |||||||||||||||||||||
| NL-CM:9.7.19932 | 0..* | Container of the Ingredient concept. This container contains all data elements of the Ingredient concept.
A product contains one or more active substances and excipients. These are usually determined by the product code. For medication prepared or compounded by the local pharmacy, each ingredient must be entered separately. The active substances play an important role, as they: a) determine the pharmacotherapeutic effect of the medication and b) serve as the basis for the indication of the strength of the medication (e.g. 200mg). |
|||||||||||||||||||||
| NL-CM:9.7.19934 | 0..1 | Active substance or excipient.
Here, the same codes can be used as for the ProductCode (for dilutions and compounds in particular), but now, the ATC, SSK and SNK codes can also be used to indicate a substance (to list ingredients of local products prepared by the pharmacy).
The ATC is an international classification of pharmaceutical substances without a reference to specific products on the market. Therefore, the ATC code of a generic product will not contain a reference to a certain dose, pharmaceutical form or route of administration; it will only contain a reference to the ingredients (not the amount/concentration/strength). |
|||||||||||||||||||||
| NL-CM:9.7.19933 | 0..1 | The relative amount of this ingredient in this product.
Calculation of Concentration = Ingredient Amount ÷ Product Amount. This could be a concentration if the medication is dissolved in liquid, for example. |
|||||||||||||||||||||
| NL-CM:9.7.22277 | 0..1 | The amount of this ingredient. This is the numerator for the calculation of the concentration. The unit should be selected from the G-Standard (Table 902). | |||||||||||||||||||||
| NL-CM:9.7.22278 | 0..1 | Amount of the product. This is the denominator for the calculation of the concentration. | |||||||||||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
| Afgesproken geneesmiddel |
| Product |
| Lisinopril tablet 10mg |
| Methotrexaat injvlst 25mg/ml 0,6 ml |
Valuesets
IngredientCodeATCCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.13 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Anatomic Therapeutic Classification (ATC) | 2.16.840.1.113883.6.73 |
IngredientCodeGPKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.9 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Generieke Product Kode (GPK) | 2.16.840.1.113883.2.4.4.1 |
IngredientCodeGTINCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.16 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Global Trade Item Number (GTIN) | 1.3.160 |
IngredientCodeHPKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.10 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
IngredientCodePRKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.11 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Voorschrijfproducten (PRK) | 2.16.840.1.113883.2.4.4.10 |
IngredientCodeSNKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.14 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-standaard Stofnaamcode (SNK) | 2.16.840.1.113883.2.4.4.1.750 |
IngredientCodeSSKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.15 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-standaard Stofnaamcode i.c.m. toedieningsweg (SSK) | 2.16.840.1.113883.2.4.4.1.725 |
IngredientCodeZICodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.12 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | 2.16.840.1.113883.2.4.4.8 |
Pharmaceutical formCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.8 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Farmaceutische vormen | 2.16.840.1.113883.2.4.4.11 |
ProductCodeATCCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.7 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Anatomic Therapeutic Classification (ATC) | 2.16.840.1.113883.6.73 |
ProductCodeGPKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.6 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Generieke Product Kode (GPK) | 2.16.840.1.113883.2.4.4.1 |
ProductCodeGTINCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.2 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | Global Trade Item Number (GTIN) | 1.3.160 |
ProductCodeHPKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.5 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Handels Product Kode (HPK) | 2.16.840.1.113883.2.4.4.7 |
ProductCodePRKCodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.3 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Voorschrijfproducten (PRK) | 2.16.840.1.113883.2.4.4.10 |
ProductCodeZICodelist
| Valueset OID 2.16.840.1.113883.2.4.3.11.60.40.2.9.7.1 |
| Conceptname | Codesystem name | Codesystem OID |
| Alle waarden | G-Standaard Artikelen (ook KNMP-nummer, ATKODE) | 2.16.840.1.113883.2.4.4.8 |
This information model in other releases
---
More on this information model
To exchange information based on health and care information models, additional, more technical specifications are required.
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Prerelease 2017 #1
Conditions for use are located on the mainpage ![]()
This page is generated on 30/11/2017 13:54:00