DAS-v1.1(2022EN): verschil tussen versies
(Nieuwe pagina aangemaakt met '<!-- Hieronder wordt een transclude page aangeroepen --> {{Versions-2.16.840.1.113883.2.4.3.11.60.40.3.12.18(EN)|1|DAS-v1.1(2022EN)}} <!-- Tot hier de transclude...') |
Geen bewerkingssamenvatting |
||
| Regel 8: | Regel 8: | ||
Release<!--hdPublication-->: '''2022''' <br> | Release<!--hdPublication-->: '''2022''' <br> | ||
Release status<!--hdPublicationStatus-->: Prepublished<br> | Release status<!--hdPublicationStatus-->: Prepublished<br> | ||
Release date<!--hdPublicationDate-->: 10- | Release date<!--hdPublicationDate-->: 15-10-2023 | ||
<!-- Aanroep Errata transclude page --> | <!-- Aanroep Errata transclude page --> | ||
{{ErrataEN<!--hdErrata-->|2022|{{PAGENAME}}}} | {{ErrataEN<!--hdErrata-->|2022|{{PAGENAME}}}} | ||
| Regel 50: | Regel 50: | ||
|style="width:250px; "|DCM::Name||nl.zorg.DAS | |style="width:250px; "|DCM::Name||nl.zorg.DAS | ||
|- | |- | ||
|style="width:250px; "|DCM::PublicationDate||10- | |style="width:250px; "|DCM::PublicationDate||15-10-2023 | ||
|- | |- | ||
|style="width:250px; "|DCM::PublicationStatus||Prepublished | |style="width:250px; "|DCM::PublicationStatus||Prepublished | ||
| Regel 73: | Regel 73: | ||
Publicatieversie <u>1.1</u> (01-12-2021) | Publicatieversie <u>1.1</u> (01-12-2021) | ||
{| | {| | ||
Issue summaries niet beschikbaar<BR> | |||
Bevat: ZIB-1248, ZIB-1404, ZIB-1508, ZIB-1567. | |||
|} | |} | ||
</div> | </div> | ||
| Regel 100: | Regel 90: | ||
<BR> | <BR> | ||
<imagemap> Bestand:DAS-v1.1Model(2022EN).png | center | <imagemap> Bestand:DAS-v1.1Model(2022EN).png | center | ||
rect 167 433 271 503 [[# | rect 167 433 271 503 [[#2435]] | ||
rect 623 122 734 183 [[# | rect 623 122 734 183 [[#2436]] | ||
rect 50 558 161 607 [[LaboratoryTestResult-v5.1(2022EN)]] | rect 50 558 161 607 [[LaboratoryTestResult-v5.1(2022EN)]] | ||
rect 170 557 281 606 [[LaboratoryTestResult-v5.1(2022EN)]] | rect 170 557 281 606 [[LaboratoryTestResult-v5.1(2022EN)]] | ||
rect 667 558 757 608 [[#LocationCodelist]] | rect 667 558 757 608 [[#LocationCodelist]] | ||
rect 665 444 755 514 [[# | rect 665 444 755 514 [[#2415]] | ||
rect 167 223 257 284 [[# | rect 167 223 257 284 [[#2430]] | ||
rect 554 447 652 517 [[AnatomicalLocation-v1.0.2(2022EN)]] | rect 554 447 652 517 [[AnatomicalLocation-v1.0.2(2022EN)]] | ||
rect 459 46 594 96 [[#DASMethodCodelist]] | rect 459 46 594 96 [[#DASMethodCodelist]] | ||
rect 319 122 430 183 [[# | rect 319 122 430 183 [[#2419]] | ||
rect 471 122 582 183 [[# | rect 471 122 582 183 [[#2420]] | ||
rect 616 323 736 393 [[# | rect 616 323 736 393 [[#2422]] | ||
rect 616 218 736 288 [[# | rect 616 218 736 288 [[#2440]] | ||
rect 167 122 278 183 [[# | rect 167 122 278 183 [[#2438]] | ||
rect 321 547 411 617 [[# | rect 321 547 411 617 [[#2418]] | ||
rect 434 548 524 618 [[# | rect 434 548 524 618 [[#2417]] | ||
rect 384 440 481 510 [[# | rect 384 440 481 510 [[#2441]] | ||
rect 391 318 481 388 [[# | rect 391 318 481 388 [[#2433]] | ||
desc none | desc none | ||
</imagemap> | </imagemap> | ||
| Regel 127: | Regel 117: | ||
|style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | |style = "text-align:center" |[[Bestand: block.png| 20px | link=]] | ||
||NL-CM:12.18.1 | ||NL-CM:12.18.1 | ||
|colspan ="6" style ="padding-left: 0px"|<span Id= | |colspan ="6" style ="padding-left: 0px"|<span Id=2433 Title="NL: DAS">[[Bestand: arrowdown.png | 10px | link=]]DAS</span> | ||
| | | | ||
|Root concept of the information model.This root concept contains all data elements of the information model DAS. | |Root concept of the information model.This root concept contains all data elements of the information model DAS. | ||
| Regel 133: | Regel 123: | ||
{| | {| | ||
|- | |- | ||
|<span title = "Codesystem: SNOMED CT; | |<span title = "Codesystem: SNOMED CT; 'Disease Activity Score' bij reumatoïde artritis (assessment scale)" >[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=443349002 443349002] Disease activity score in rheumatoid arthritis</span> | ||
|} | |} | ||
| | | | ||
| Regel 140: | Regel 130: | ||
||NL-CM:12.18.15 | ||NL-CM:12.18.15 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2419 Title="NL: DASWaarde">[[Bestand: arrowright.png | 10px | link=]]DASValue</span> | ||
|0..1 | |0..1 | ||
|The result (value). | |The result (value). | ||
| Regel 153: | Regel 143: | ||
||NL-CM:12.18.14 | ||NL-CM:12.18.14 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2420 Title="NL: DASMethode">[[Bestand: arrowright.png | 10px | link=]]DASMethod</span> | ||
|1 | |1 | ||
|The specification of the DAS method used. | |The specification of the DAS method used. | ||
| Regel 160: | Regel 150: | ||
{| | {| | ||
|- | |- | ||
|<span title = "Codesystem: SNOMED CT; | |<span title = "Codesystem: SNOMED CT; 'Disease Activity Score' bij reumatoïde artritis (assessment scale)" >[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=443349002 443349002] Disease activity score in rheumatoid arthritis</span> | ||
|} | |} | ||
| | | | ||
| Regel 171: | Regel 161: | ||
||NL-CM:12.18.21 | ||NL-CM:12.18.21 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2436 Title="NL: DASFormule">[[Bestand: arrowright.png | 10px | link=]]DASFormula</span> | ||
|0..1 | |0..1 | ||
|The specification of the type of DAS. | |The specification of the type of DAS. | ||
| Regel 181: | Regel 171: | ||
||NL-CM:12.18.2 | ||NL-CM:12.18.2 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2441 Title="NL: AangedaanGewricht">[[Bestand: arrowdown.png | 10px | link=]]AffectedJoint</span> | ||
|0..* | |0..* | ||
|Container of the AffectedJoint concept.This container contains all data elements of the AffectedJoint concept. | |Container of the AffectedJoint concept.This container contains all data elements of the AffectedJoint concept. | ||
| Regel 195: | Regel 185: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Id= | |colspan ="4" style ="padding-left: 0px"|<span Id=2437 Title="NL: AnatomischeLocatie">[[Bestand: arrowright.png | 10px | link=]]AnatomicalLocation</span> | ||
|0..1 | |0..1 | ||
|Joints that are tender and/or swollen. | |Joints that are tender and/or swollen. | ||
| Regel 211: | Regel 201: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="2" style ="padding-left: 0px"|<span Id= | |colspan ="2" style ="padding-left: 0px"|<span Id=2415 Title="NL: Locatie">[[Bestand: arrowright.png | 10px | link=]]Location</span> | ||
| | | | ||
|The location and laterality of joints in the body. | |The location and laterality of joints in the body. | ||
| Regel 229: | Regel 219: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Id= | |colspan ="4" style ="padding-left: 0px"|<span Id=2418 Title="NL: Gezwollen">[[Bestand: arrowright.png | 10px | link=]]Swollen</span> | ||
|0..1 | |0..1 | ||
|Swolen joint indicator. | |Swolen joint indicator. | ||
| Regel 243: | Regel 233: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Id= | |colspan ="4" style ="padding-left: 0px"|<span Id=2417 Title="NL: Pijnlijk">[[Bestand: arrowright.png | 10px | link=]]Tender</span> | ||
|0..1 | |0..1 | ||
|Tender joint indicator. | |Tender joint indicator. | ||
| Regel 256: | Regel 246: | ||
||NL-CM:12.18.11 | ||NL-CM:12.18.11 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2440 Title="NL: AantalPijnlijkeGewrichten">[[Bestand: arrowright.png | 10px | link=]]CountOfTenderJoints</span> | ||
|0..1 | |0..1 | ||
|Count of tender joints as reported by the patient. | |Count of tender joints as reported by the patient.  | ||
| | | | ||
{| | {| | ||
|- | |- | ||
|<span title = "Codesystem: SNOMED CT; Aantal gevoelige gewrichten voor evaluatie van | |<span title = "Codesystem: SNOMED CT; Aantal gevoelige gewrichten voor evaluatie van reumatoïde artritis (observable entity)" >[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=442521001 442521001] Tender joint count for rheumatoid arthritis assessment</span> | ||
|} | |} | ||
| | | | ||
| Regel 269: | Regel 259: | ||
||NL-CM:12.18.12 | ||NL-CM:12.18.12 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2422 Title="NL: AantalGezwollenGewrichten">[[Bestand: arrowright.png | 10px | link=]]CountOfSwolenJoints</span> | ||
|0..1 | |0..1 | ||
|Count of swollen joints. | |Count of swollen joints. | ||
| Regel 275: | Regel 265: | ||
{| | {| | ||
|- | |- | ||
|<span title = "Codesystem: SNOMED CT; Aantal gezwollen gewrichten voor evaluatie van | |<span title = "Codesystem: SNOMED CT; Aantal gezwollen gewrichten voor evaluatie van reumatoïde artritis (observable entity)" >[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=442194005 442194005] Swollen joint count for rheumatoid arthritis assessment</span> | ||
|} | |} | ||
| | | | ||
| Regel 282: | Regel 272: | ||
||NL-CM:12.18.22 | ||NL-CM:12.18.22 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2435 Title="NL: OntstekingIndicator">[[Bestand: arrowdown.png | 10px | link=]]InflammationIndicator</span> | ||
|0..1 | |0..1 | ||
|Container of the InflammationIndicator concept.This container contains all data elements of the InflammationIndicator concept. | |Container of the InflammationIndicator concept.This container contains all data elements of the InflammationIndicator concept. | ||
| Regel 292: | Regel 282: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Id= | |colspan ="4" style ="padding-left: 0px"|<span Id=2442 Title="NL: BSE::LaboratoriumUitslag">[[Bestand: arrowright.png | 10px | link=]]ESR::LaboratoryResult</span> | ||
|(0..1) | |(0..1) | ||
|The C-reactive protein (CRP) is a laboratory measurment valuable for determining the presence of inflammation or to monitor the effect of a medical treatment on the inflammation. The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube over a period of one hour. Both test results can be used to calculate the DAS. | |The C-reactive protein (CRP) is a laboratory measurment valuable for determining the presence of inflammation or to monitor the effect of a medical treatment on the inflammation. The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube over a period of one hour. Both test results can be used to calculate the DAS.  | ||
| | | | ||
{| | {| | ||
| Regel 312: | Regel 302: | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: none;" | | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="4" style ="padding-left: 0px"|<span Id= | |colspan ="4" style ="padding-left: 0px"|<span Id=2439 Title="NL: CRP::LaboratoriumUitslag">[[Bestand: arrowright.png | 10px | link=]]CRP::LaboratoryResult</span> | ||
|(0..1) | |(0..1) | ||
|The C-reactive protein (CRP) is a laboratory measurment valuable for determining the presence of inflammation or to monitor the effect of a medical treatment on the inflammation. The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube over a period of one hour. Both test results can be used to calculate the DAS. | |The C-reactive protein (CRP) is a laboratory measurment valuable for determining the presence of inflammation or to monitor the effect of a medical treatment on the inflammation. The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube over a period of one hour. Both test results can be used to calculate the DAS.  | ||
| | | | ||
{| | {| | ||
|- | |- | ||
|<span title = "Codesystem: LOINC; C reactief | |<span title = "Codesystem: LOINC; C reactief proteïne [massa/volume] in serum of plasma (Qn)" >[https://terminologie.nictiz.nl/art-decor/loinc?conceptId=1988-5 1988-5] C reactive protein [Mass/volume] in Serum or Plasma</span> | ||
|- | |- | ||
|<span title = "Codesystem: LOINC; C reactief | |<span title = "Codesystem: LOINC; C reactief proteïne [massa/volume] in serum of plasma (Qn)" >[https://terminologie.nictiz.nl/art-decor/loinc?conceptId=30522-7 30522-7] C reactive protein [Mass/volume] in Serum or Plasma by High sensitivity method</span> | ||
|} | |} | ||
| | | | ||
| Regel 331: | Regel 321: | ||
||NL-CM:12.18.17 | ||NL-CM:12.18.17 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2430 Title="NL: DASDatumTijd">[[Bestand: arrowright.png | 10px | link=]]DASDateTime</span> | ||
|0..1 | |0..1 | ||
|The date and possibly time when the DAS score measurement was carried out. | |The date and possibly time when the DAS score measurement was carried out. | ||
| Regel 344: | Regel 334: | ||
||NL-CM:12.18.8 | ||NL-CM:12.18.8 | ||
|style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | |style = "width: 7px; padding-left: 0px; padding-right: 0px; border-left: none; border-right: 1px dotted silver;" | | ||
|colspan ="5" style ="padding-left: 0px"|<span Id= | |colspan ="5" style ="padding-left: 0px"|<span Id=2438 Title="NL: VASAlgemeneGezondheid">[[Bestand: arrowright.png | 10px | link=]]VASGeneralHealth</span> | ||
|0..1 | |0..1 | ||
|The VAS score for general health as experienced by the patient on a scale of 1 to 100 | |The VAS score for general health as experienced by the patient on a scale of 1 to 100 | ||
| Regel 360: | Regel 350: | ||
''Only available in Dutch<!--noTranslation-->'' | ''Only available in Dutch<!--noTranslation-->'' | ||
Voorbeeld file fout: Unable to find the specified file. : nl.zorg.DAS-v1.1(NL)_Voorbeeld.docx | |||
==References== | ==References== | ||
Informatie over het DAS instrument [Online] Beschikbaar op: https://www.das-score.nl/das28/en/ [Geraadpleegd: 8 oktober 2019] | Informatie over het DAS instrument [Online] Beschikbaar op: https://www.das-score.nl/das28/en/ [Geraadpleegd: 8 oktober 2019] | ||
| Regel 520: | Regel 368: | ||
|Description<!--vsDescription--> | |Description<!--vsDescription--> | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "(assessment scale)">DAS28 - CRP</span> | |<span title = "DAS28 - CRP (assessment scale)">DAS28 - CRP</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=117651000146103 117651000146103] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=117651000146103 117651000146103] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 532: | Regel 380: | ||
|DAS28-BSE | |DAS28-BSE | ||
|-style="vertical-align:top; " | |-style="vertical-align:top; " | ||
|<span title = "(assessment scale)">DAS44 - CRP</span> | |<span title = "DAS44 - CRP (assessment scale)">DAS44 - CRP</span> | ||
|[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=117681000146108 117681000146108] | |[https://terminologie.nictiz.nl/art-decor/snomed-ct?conceptId=117681000146108 117681000146108] | ||
|SNOMED CT | |SNOMED CT | ||
| Regel 809: | Regel 657: | ||
:-- | :-- | ||
==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | ==Technical specifications in HL7v3 CDA and HL7 FHIR<!--ftHeader-->== | ||
To exchange information based on health and care information models, additional, more technical specifications are required. | To exchange information based on health and care information models, additional, more technical specifications are required.<BR> | ||
Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:<!--ftReferenceIntro--> | Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:<!--ftReferenceIntro--> | ||
<ul> | <ul> | ||
<li> | <li>HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment<!--ftArtDecorReference--> {{ArtDecorLinks|2022|12.18}}</li> | ||
<li> | <li>HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR<!--ftSimplifierReference--> {{SimplefierLinks|2022|DAS}}</li> | ||
</ul> | </ul> | ||
==Downloads<!--ftDownloadTitle-->== | ==Downloads<!--ftDownloadTitle-->== | ||
| Regel 821: | Regel 669: | ||
SNOMED CT and LOINC codes are based on: | SNOMED CT and LOINC codes are based on: | ||
<ul> | <ul> | ||
<li>SNOMED Clinical Terms | <li>SNOMED Clinical Terms versie: 20230930 [R] (september 2023-editie)</li> | ||
<li>LOINC version 2. | <li>LOINC version 2.76</li> | ||
</ul> | </ul> | ||
Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | Conditions for use are located on the mainpage<!--ftConditions--> [[Bestand:list2.png|link=HCIM_Mainpage<!--wikiMainpage-->]]<BR> | ||
This page is generated on | This page is generated on 26/03/2024 11:41:17 with ZibExtraction v. 9.3.8851.20230<!--ftDate--> <BR> | ||
----- | ----- | ||
<div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2022(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2022(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | <div style="text-align: right; direction: ltr; margin-left: 1em;" >[[Bestand: Back 16.png| link= HCIM_Release<!--wikiReleasePage-->_2022(EN)]] [[HCIM_Release<!--wikiReleasePage-->_2022(EN) |Back to HCIM list<!--hdBackToMainPage--> ]]</div> | ||
Versie van 26 mrt 2024 11:47
General information
Name: nl.zorg.DAS ![]()
Version: 1.1
HCIM Status:Final
Release: 2022
Release status: Prepublished
Release date: 15-10-2023
Metadata
| DCM::CoderList | * |
| DCM::ContactInformation.Address | * |
| DCM::ContactInformation.Name | * |
| DCM::ContactInformation.Telecom | * |
| DCM::ContentAuthorList | * |
| DCM::CreationDate | 26-03-2020 |
| DCM::DeprecatedDate | |
| DCM::DescriptionLanguage | nl |
| DCM::EndorsingAuthority.Address | |
| DCM::EndorsingAuthority.Name | * |
| DCM::EndorsingAuthority.Telecom | |
| DCM::Id | 2.16.840.1.113883.2.4.3.11.60.40.3.12.18 |
| DCM::KeywordList | |
| DCM::LifecycleStatus | Final |
| DCM::ModelerList | Linda Mook |
| DCM::Name | nl.zorg.DAS |
| DCM::PublicationDate | 15-10-2023 |
| DCM::PublicationStatus | Prepublished |
| DCM::ReviewerList | |
| DCM::RevisionDate | 28-07-2021 |
| DCM::Supersedes | nl.zorg.DAS-v1.0 |
| DCM::Version | 1.1 |
| HCIM::PublicationLanguage | EN |
Revision History
Only available in Dutch
Publicatieversie 1.0 (01-09-2020)
Publicatieversie 1.1 (01-12-2021)
Issue summaries niet beschikbaarBevat: ZIB-1248, ZIB-1404, ZIB-1508, ZIB-1567.
Concept
The Disease Activity Score (DAS) is a combined index developed in Nijmegen around 1990 to measure disease activity in patients with rheumatoid arthritis (RA). It concerns a formula with 28 or 44 painful and swollen joints, a blood value for inflammation (BSE or CRP) and the overall experience of disease activity by the patient. The DAS results in a number between 0 and 10, which indicates how active rheumatoid arthritis is at present. The score has been validated for use in clinical trials in combination with the EULAR response criteria.
Purpose
In addition to frequent use in trials, the DAS is used to measure the disease activity of RA patients in daily clinical practice, supporting the "treat-to-target" strategy recommended in guidelines. The DAS is also used as an outcome measure for the care of RA patients in a national quality registration.
Patient Population
Adult patients with rheumatoid arthritis (RA).
Information Model
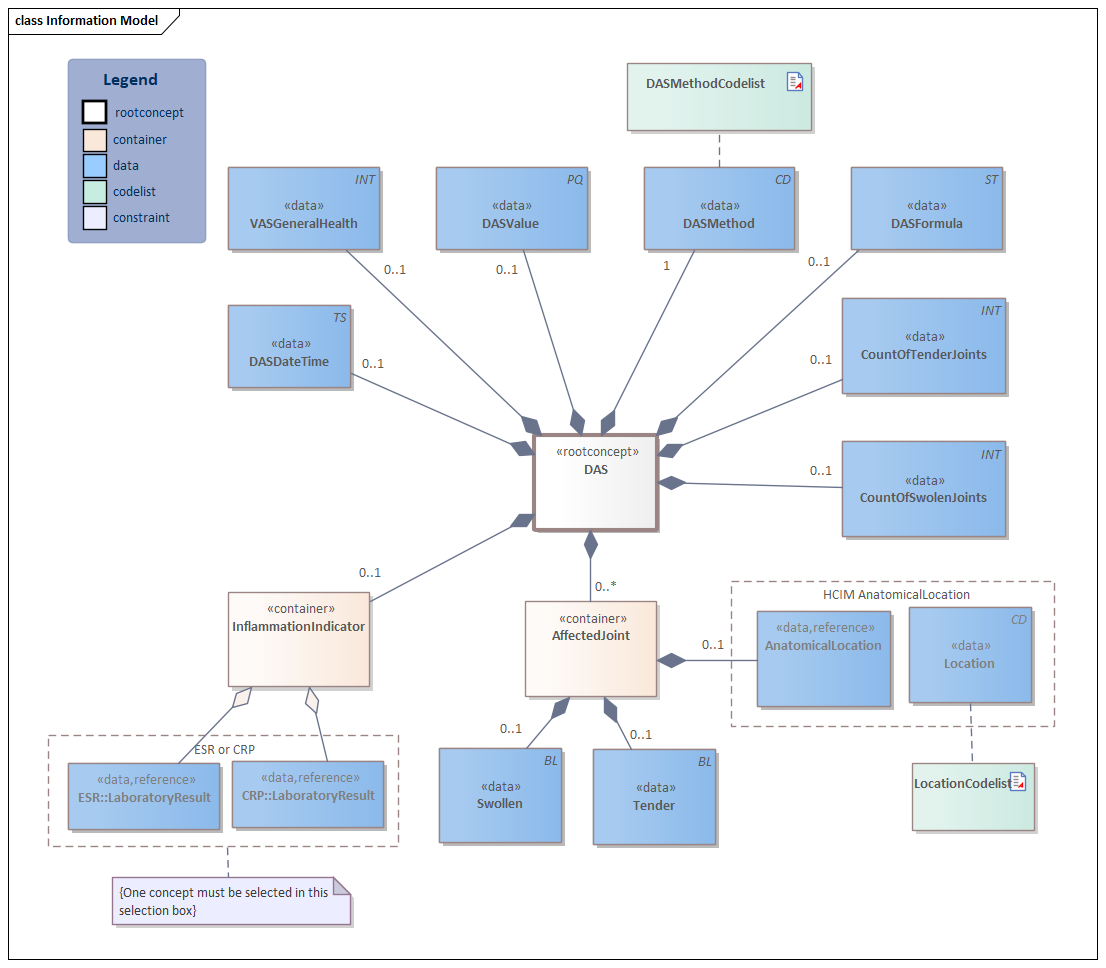
| Type | Id | Concept | Card. | Definition | DefinitionCode | Reference | |||||||||
| NL-CM:12.18.1 | Root concept of the information model.This root concept contains all data elements of the information model DAS. |
|
|||||||||||||
| NL-CM:12.18.15 | 0..1 | The result (value). |
|
||||||||||||
| NL-CM:12.18.14 | 1 | The specification of the DAS method used.
There are different DAS scores. Each type has a different formula. DAS-28 or DAS-44 stands for 28 or 44 painful and swollen joints. One blood value for inflammation (a ESR or a CRP laboratory result) and the overall experience of disease activity by the patient via a Visual Analog Scale (VAS). |
|
| |||||||||||
| NL-CM:12.18.21 | 0..1 | The specification of the type of DAS.
There are different DAS scores. Each type has a different formula. |
|||||||||||||
| NL-CM:12.18.2 | 0..* | Container of the AffectedJoint concept.This container contains all data elements of the AffectedJoint concept. |
|
||||||||||||
| NL-CM:12.18.16 | 0..1 | Joints that are tender and/or swollen. |
| ||||||||||||
| NL-CM:20.7.4 | The location and laterality of joints in the body. |
|
| ||||||||||||
| NL-CM:12.18.4 | 0..1 | Swolen joint indicator. |
|
||||||||||||
| NL-CM:12.18.3 | 0..1 | Tender joint indicator. |
|
||||||||||||
| NL-CM:12.18.11 | 0..1 | Count of tender joints as reported by the patient. |
|
||||||||||||
| NL-CM:12.18.12 | 0..1 | Count of swollen joints. |
|
||||||||||||
| NL-CM:12.18.22 | 0..1 | Container of the InflammationIndicator concept.This container contains all data elements of the InflammationIndicator concept. | |||||||||||||
| NL-CM:12.18.20 | (0..1) | The C-reactive protein (CRP) is a laboratory measurment valuable for determining the presence of inflammation or to monitor the effect of a medical treatment on the inflammation. The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube over a period of one hour. Both test results can be used to calculate the DAS. |
|
| |||||||||||
| NL-CM:12.18.19 | (0..1) | The C-reactive protein (CRP) is a laboratory measurment valuable for determining the presence of inflammation or to monitor the effect of a medical treatment on the inflammation. The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube over a period of one hour. Both test results can be used to calculate the DAS. |
|
| |||||||||||
| NL-CM:12.18.17 | 0..1 | The date and possibly time when the DAS score measurement was carried out. |
|
||||||||||||
| NL-CM:12.18.8 | 0..1 | The VAS score for general health as experienced by the patient on a scale of 1 to 100 |
|
||||||||||||
Columns Concept and DefinitionCode: hover over the values for more information
For explanation of the symbols, please see the legend page ![]()
Example Instances
Only available in Dutch
Voorbeeld file fout: Unable to find the specified file. : nl.zorg.DAS-v1.1(NL)_Voorbeeld.docx
References
Informatie over het DAS instrument [Online] Beschikbaar op: https://www.das-score.nl/das28/en/ [Geraadpleegd: 8 oktober 2019]
Valuesets
DASMethodCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.12.18.2 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| DAS28 - CRP | 117651000146103 | SNOMED CT | 2.16.840.1.113883.6.96 | DAS 28-CRP |
| DAS28 - ESR | 117661000146100 | SNOMED CT | 2.16.840.1.113883.6.96 | DAS28-BSE |
| DAS44 - CRP | 117681000146108 | SNOMED CT | 2.16.840.1.113883.6.96 | DAS44-CRP |
| DAS44 - ESR | 117691000146105 | SNOMED CT | 2.16.840.1.113883.6.96 | DAS44-BSE |
LocationCodelist
| Valueset OID: 2.16.840.1.113883.2.4.3.11.60.40.2.12.18.1 | Binding: Extensible |
| Conceptname | Conceptcode | Codesystem name | Codesystem OID | Description |
| Temporomandibular joint structure | 53620006 | SNOMED CT | 2.16.840.1.113883.6.96 | Kaakgewricht |
| Sternoclavicular joint structure | 7844006 | SNOMED CT | 2.16.840.1.113883.6.96 | Sternoclaviculair gewricht |
| Acromioclavicular joint structure | 85856004 | SNOMED CT | 2.16.840.1.113883.6.96 | Acromioclaviculair gewricht |
| Glenohumeral joint structure | 85537004 | SNOMED CT | 2.16.840.1.113883.6.96 | Schoudergewricht |
| Elbow joint structure | 16953009 | SNOMED CT | 2.16.840.1.113883.6.96 | Ellebooggewricht |
| Wrist joint structure | 74670003 | SNOMED CT | 2.16.840.1.113883.6.96 | Polsgewricht |
| Carpometacarpal joint structure of thumb | 30099006 | SNOMED CT | 2.16.840.1.113883.6.96 | Carpometacarpaal gewricht van duim |
| Metacarpophalangeal joint structure of thumb | 56310002 | SNOMED CT | 2.16.840.1.113883.6.96 | Metacarpofalangeaal gewricht van duim |
| Metacarpophalangeal joint structure of index finger | 289002 | SNOMED CT | 2.16.840.1.113883.6.96 | Metacarpofalangeaal gewricht van wijsvinger |
| Metacarpophalangeal joint structure of middle finger | 6059006 | SNOMED CT | 2.16.840.1.113883.6.96 | Metacarpofalangeaal gewricht van middelvinger |
| Metacarpophalangeal joint structure of ring finger | 13822002 | SNOMED CT | 2.16.840.1.113883.6.96 | Metacarpofalangeaal gewricht van ringvinger |
| Metacarpophalangeal joint structure of little finger | 5493005 | SNOMED CT | 2.16.840.1.113883.6.96 | Metacarpofalangeaal gewricht van pink |
| Structure of interphalangeal joint of thumb | 115955008 | SNOMED CT | 2.16.840.1.113883.6.96 | Interfalangeaal gewricht van duim |
| Structure of proximal interphalangeal joint of index finger | 371270005 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van wijsvinger |
| Structure of proximal interphalangeal joint of middle finger | 371263005 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van middelvinger |
| Structure of proximal interphalangeal joint of ring finger | 371267006 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van ringvinger |
| Structure of proximal interphalangeal joint of little finger | 371233001 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van pink |
| Structure of distal interphalangeal joint of index finger | 371210002 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van wijsvinger |
| Structure of distal interphalangeal joint of middle finger | 371218009 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van middelvinger |
| Structure of distal interphalangeal joint of ring finger | 371204002 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van ringvinger |
| Structure of distal interphalangeal joint of little finger | 371202003 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van pink |
| Sacroiliac joint structure | 39723000 | SNOMED CT | 2.16.840.1.113883.6.96 | Sacroiliacaal gewricht |
| Hip joint structure | 24136001 | SNOMED CT | 2.16.840.1.113883.6.96 | Heupgewricht |
| Knee joint structure | 49076000 | SNOMED CT | 2.16.840.1.113883.6.96 | Kniegewricht |
| Ankle joint structure | 70258002 | SNOMED CT | 2.16.840.1.113883.6.96 | Enkelgewricht |
| Intertarsal joint structure | 27949001 | SNOMED CT | 2.16.840.1.113883.6.96 | structuur van articulatio intertarsalis |
| First metatarsophalangeal joint structure | 27824001 | SNOMED CT | 2.16.840.1.113883.6.96 | Metatarsofalangeaal gewricht van grote teen |
| Metatarsophalangeal joint structure of second toe | 71721008 | SNOMED CT | 2.16.840.1.113883.6.96 | Metatarsofalangeaal gewricht van tweede teen |
| Metatarsophalangeal joint structure of third toe | 81247008 | SNOMED CT | 2.16.840.1.113883.6.96 | Metatarsofalangeaal gewricht van derde teen |
| Metatarsophalangeal joint structure of fourth toe | 45230000 | SNOMED CT | 2.16.840.1.113883.6.96 | Metatarsofalangeaal gewricht van vierde teen |
| Metatarsophalangeal joint structure of fifth toe | 50133002 | SNOMED CT | 2.16.840.1.113883.6.96 | Metatarsofalangeaal gewricht van kleine teen |
| Interphalangeal joint structure of great toe | 363670009 | SNOMED CT | 2.16.840.1.113883.6.96 | Interfalangeaal gewricht van grote teen |
| Proximal interphalangeal joint structure of second toe | 371292009 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van tweede teen |
| Proximal interphalangeal joint structure of third toe | 371255009 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van derde teen |
| Proximal interphalangeal joint structure of fourth toe | 371288002 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van vierde teen |
| Proximal interphalangeal joint structure of fifth toe | 371284000 | SNOMED CT | 2.16.840.1.113883.6.96 | Proximaal interfalangeaal gewricht van kleine (vijfde) teen |
| Distal interphalangeal joint structure of second toe | 371216008 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van tweede teen |
| Distal interphalangeal joint structure of third toe | 371219001 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van derde teen |
| Distal interphalangeal joint structure of fourth toe | 371205001 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van vierde teen |
| Distal interphalangeal joint structure of fifth toe | 371203008 | SNOMED CT | 2.16.840.1.113883.6.96 | Distaal interfalangeaal gewricht van kleine (vijfde) teen |
This information model in other releases
- Release 2020, (Version 1.0)
- Prerelease 2021-2, (Version 1.1)
- Prerelease 2023-1, (Version 1.1)
- Prerelease 2024-1, (Version 1.1)
Information model references
This information model refers to
This information model is used in
- --
Technical specifications in HL7v3 CDA and HL7 FHIR
To exchange information based on health and care information models, additional, more technical specifications are required.<BR> Not every environment can handle the same technical specifications. For this reason, there are several types of technical specifications:
- HL7® version 3 CDA compatible specifications, available through the Nictiz ART-DECOR® environment

- HL7® FHIR® compatible specifications, available through the Nictiz environment on the Simplifier FHIR

Downloads
This information model is also available as pdf file ![]() or as spreadsheet
or as spreadsheet ![]()
About this information
The information in this wikipage is based on Pre-release 2022-1
SNOMED CT and LOINC codes are based on:
- SNOMED Clinical Terms versie: 20230930 [R] (september 2023-editie)
- LOINC version 2.76
Conditions for use are located on the mainpage ![]()
This page is generated on 26/03/2024 11:41:17 with ZibExtraction v. 9.3.8851.20230